Dislocations Linear Defects Twodimensional or line defect Line

- Slides: 18

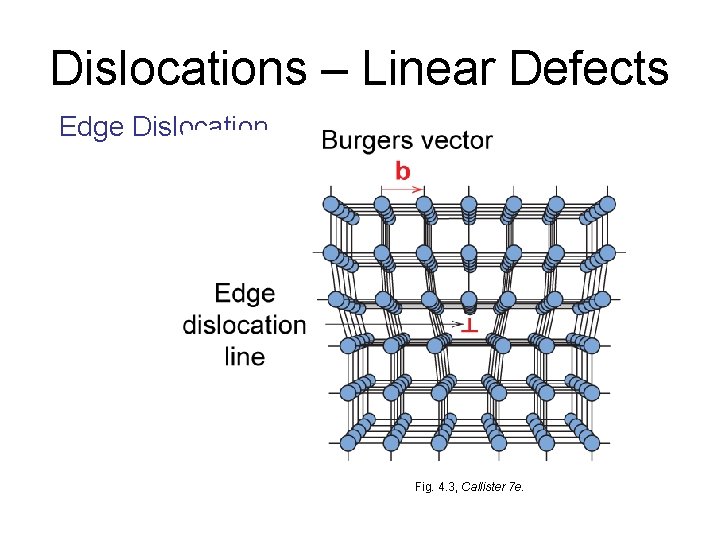
Dislocations – Linear Defects – Two-dimensional or line defect – Line around which atoms are misaligned – related to slip • Edge dislocation: – extra half-plane of atoms inserted in a crystal structure – Or – think of it as a partially slipped crystal – b to dislocation line • Screw dislocation: – spiral planar ramp resulting from shear deformation – b to dislocation line Burger’s vector, b: measure of lattice distortion or the amount of displacement. Burger’s vector is equal in magnitude to interatomic spacing.

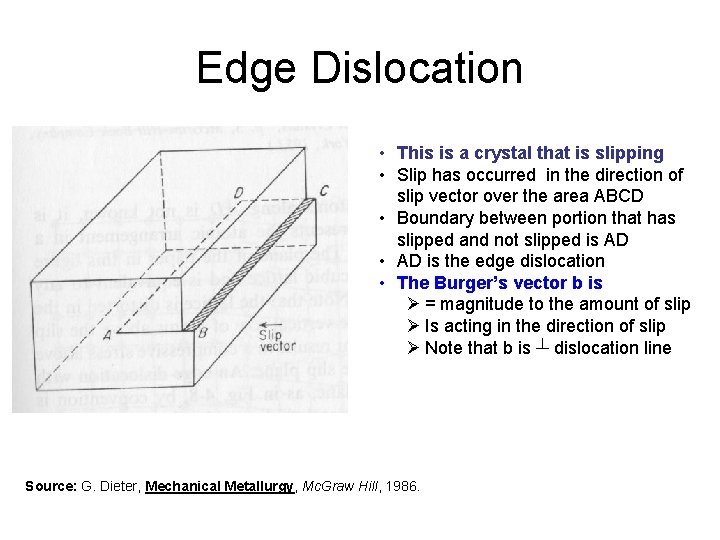
Edge Dislocation • This is a crystal that is slipping • Slip has occurred in the direction of slip vector over the area ABCD • Boundary between portion that has slipped and not slipped is AD • AD is the edge dislocation • The Burger’s vector b is Ø = magnitude to the amount of slip Ø Is acting in the direction of slip Ø Note that b is ┴ dislocation line Source: G. Dieter, Mechanical Metallurgy, Mc. Graw Hill, 1986.

Dislocations – Linear Defects Edge Dislocation Fig. 4. 3, Callister 7 e.

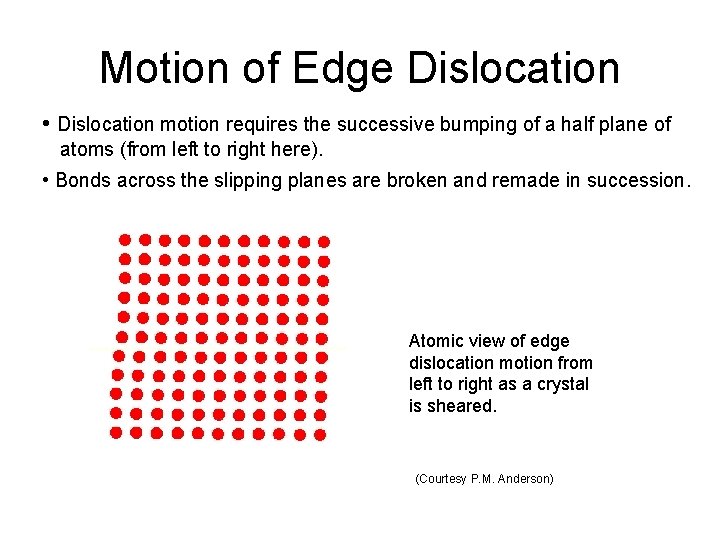
Motion of Edge Dislocation • Dislocation motion requires the successive bumping of a half plane of atoms (from left to right here). • Bonds across the slipping planes are broken and remade in succession. Atomic view of edge dislocation motion from left to right as a crystal is sheared. (Courtesy P. M. Anderson)

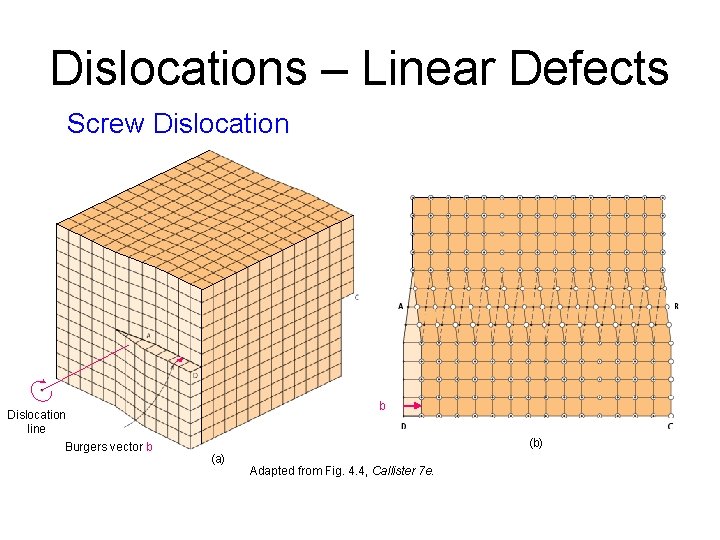
Dislocations – Linear Defects Screw Dislocation line Burgers vector b b (b) (a) Adapted from Fig. 4. 4, Callister 7 e.

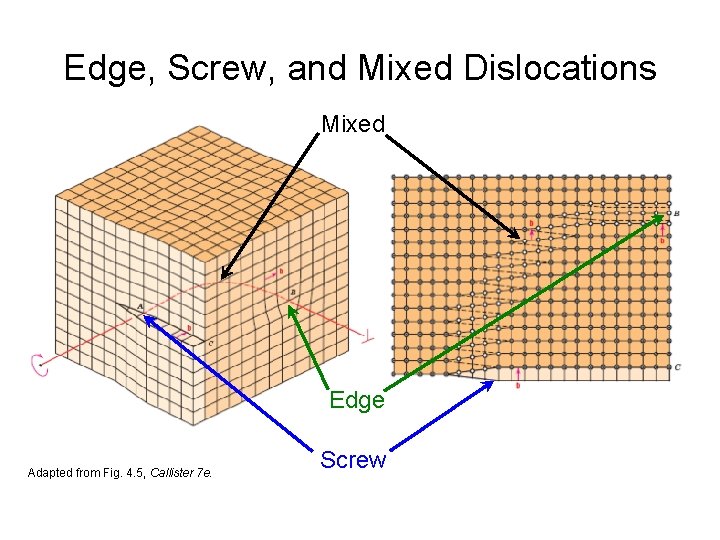
Edge, Screw, and Mixed Dislocations Mixed Edge Adapted from Fig. 4. 5, Callister 7 e. Screw

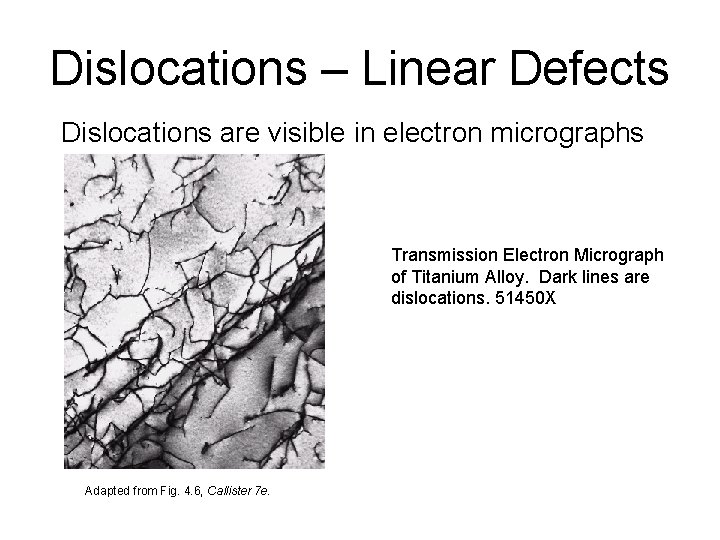
Dislocations – Linear Defects Dislocations are visible in electron micrographs Transmission Electron Micrograph of Titanium Alloy. Dark lines are dislocations. 51450 X Adapted from Fig. 4. 6, Callister 7 e.

Interfacial - Planar Defects Surfaces – – Atoms do not have the same coordination number Therefore are in higher energy state Surface energy, g [=] J/m 2 Materials always try to reduce surface energy – tendency towards spherical shapes

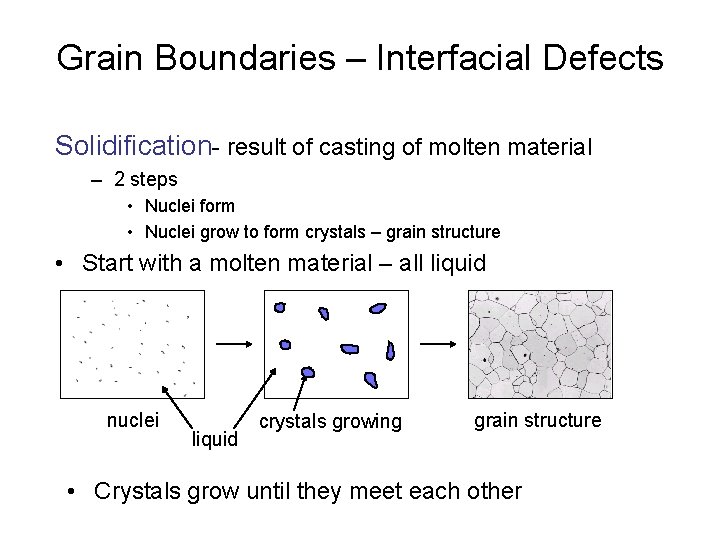
Grain Boundaries – Interfacial Defects Solidification- result of casting of molten material – 2 steps • Nuclei form • Nuclei grow to form crystals – grain structure • Start with a molten material – all liquid nuclei liquid crystals growing grain structure • Crystals grow until they meet each other

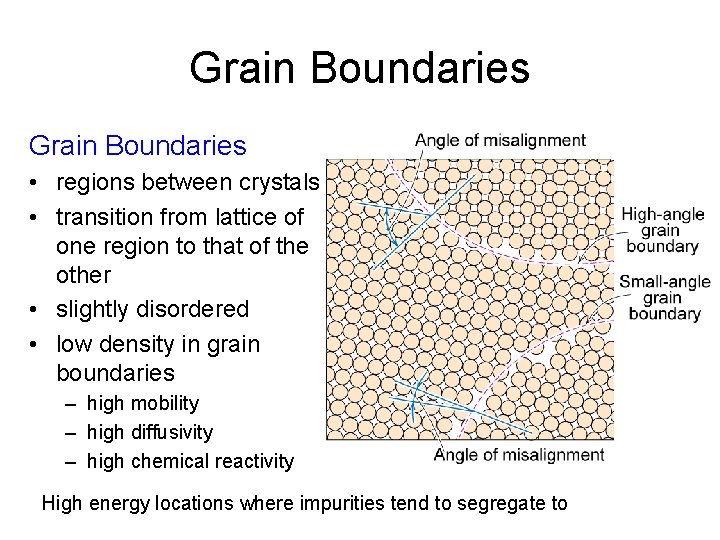
Grain Boundaries • regions between crystals • transition from lattice of one region to that of the other • slightly disordered • low density in grain boundaries – high mobility – high diffusivity – high chemical reactivity High energy locations where impurities tend to segregate to

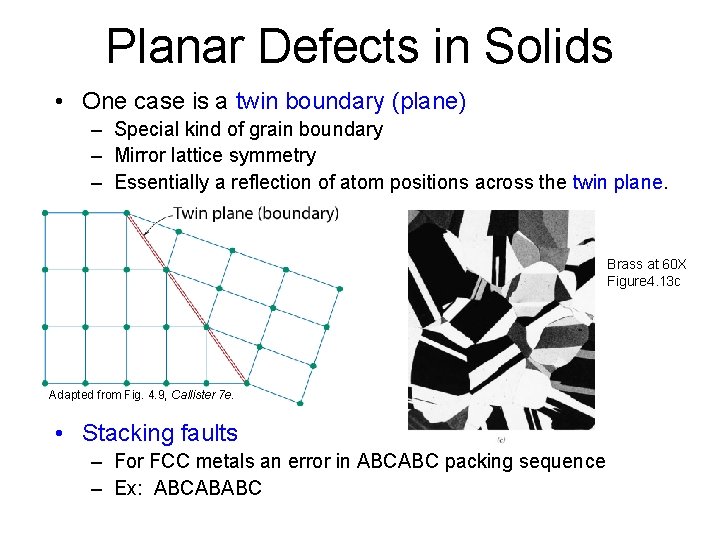
Planar Defects in Solids • One case is a twin boundary (plane) – Special kind of grain boundary – Mirror lattice symmetry – Essentially a reflection of atom positions across the twin plane. Brass at 60 X Figure 4. 13 c Adapted from Fig. 4. 9, Callister 7 e. • Stacking faults – For FCC metals an error in ABCABC packing sequence – Ex: ABCABABC

Diffusion - Mass transport by atomic motion Mechanisms • Gases & Liquids – random (Brownian) motion • Solids – vacancy diffusion or interstitial diffusion

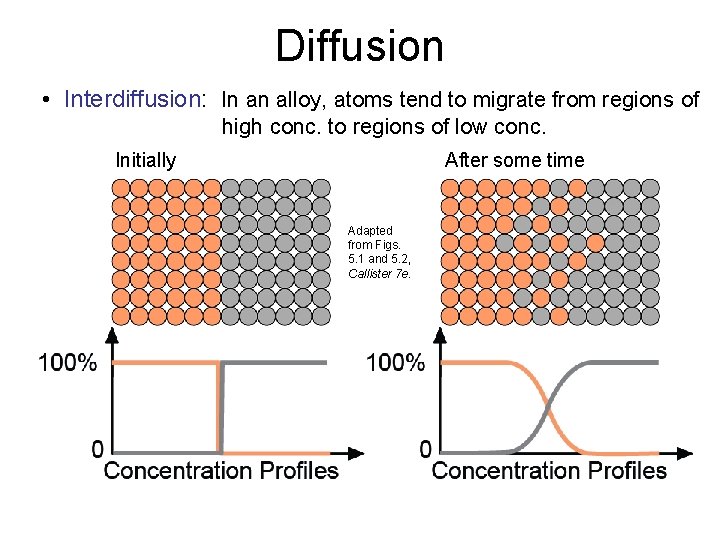
Diffusion • Interdiffusion: In an alloy, atoms tend to migrate from regions of high conc. to regions of low conc. Initially After some time Adapted from Figs. 5. 1 and 5. 2, Callister 7 e.

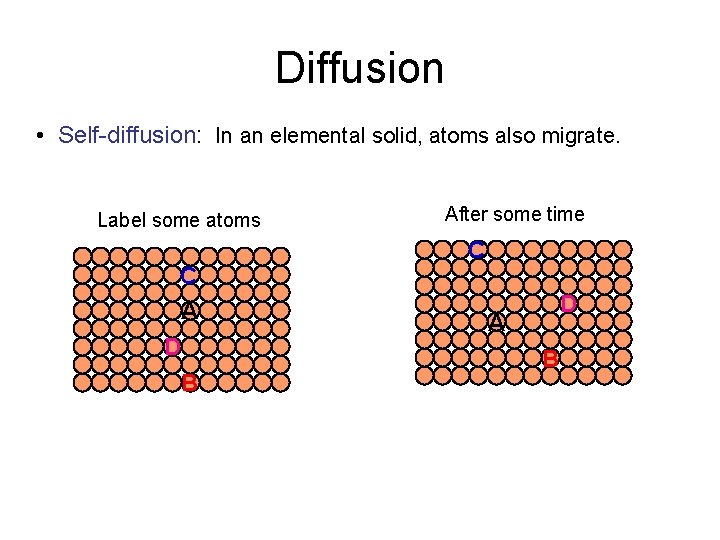
Diffusion • Self-diffusion: In an elemental solid, atoms also migrate. Label some atoms C A D B After some time C D A B

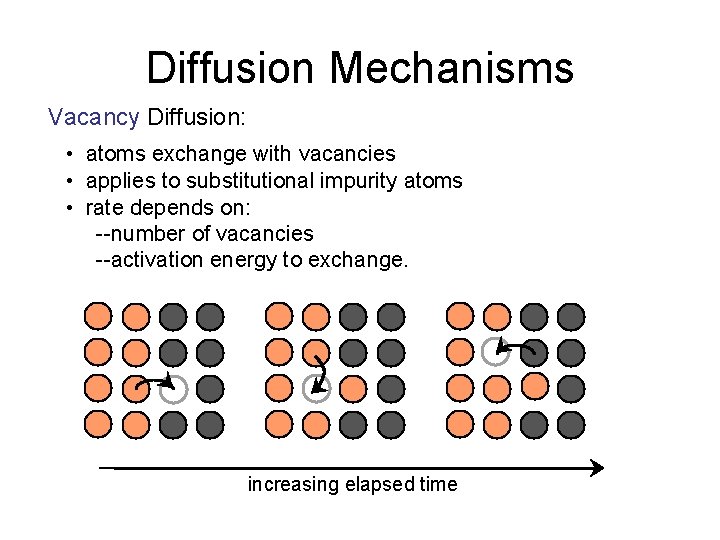
Diffusion Mechanisms Vacancy Diffusion: • atoms exchange with vacancies • applies to substitutional impurity atoms • rate depends on: --number of vacancies --activation energy to exchange. increasing elapsed time

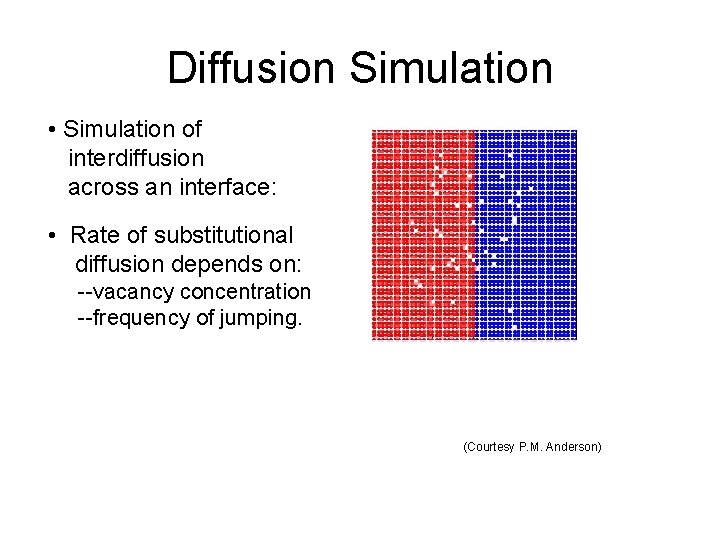
Diffusion Simulation • Simulation of interdiffusion across an interface: • Rate of substitutional diffusion depends on: --vacancy concentration --frequency of jumping. (Courtesy P. M. Anderson)

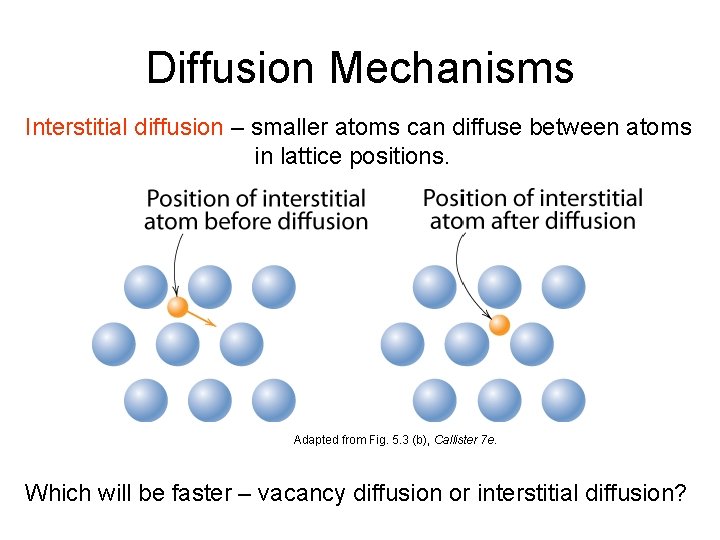
Diffusion Mechanisms Interstitial diffusion – smaller atoms can diffuse between atoms in lattice positions. Adapted from Fig. 5. 3 (b), Callister 7 e. Which will be faster – vacancy diffusion or interstitial diffusion?

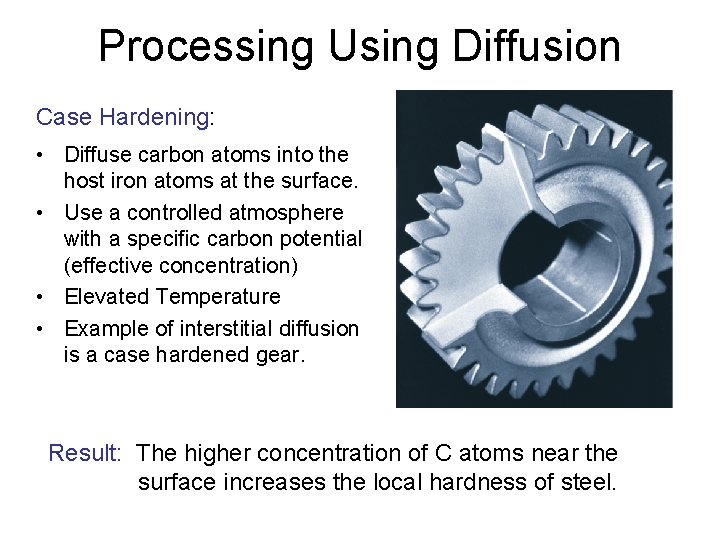
Processing Using Diffusion Case Hardening: • Diffuse carbon atoms into the host iron atoms at the surface. • Use a controlled atmosphere with a specific carbon potential (effective concentration) • Elevated Temperature • Example of interstitial diffusion is a case hardened gear. Result: The higher concentration of C atoms near the surface increases the local hardness of steel.