Disinfection Sterilization and Antisepsis An Overview William A






























- Slides: 41

Disinfection, Sterilization and Antisepsis: An Overview William A. Rutala, Ph. D, MPH Director, Hospital Epidemiology, Occupational Health and Safety; Research Professor of Medicine and Director, Statewide Program for Infection Control and Epidemiology University of North Carolina at Chapel Hill and UNC Health Care, Chapel Hill, NC

DISCLOSURES • Consultation n ASP (Advanced Sterilization Products)-2014 n Clorox-2014, 2015 • Honoraria (2014, 2015) § 3 M, ASP, Clorox • Grants n CDC, CMS, Nanosonics

Disinfection, Sterilization and Antisepsis Ä Provide overview of disinfection, sterilization and antisepsis n Indications and methods for sterilization, high-level disinfection and low-level disinfection n Cleaning of patient-care devices n Sterilization n Disinfection (high-level and low-level disinfection)

CDC Guideline for Disinfection and Sterilization Rutala, Weber, HICPAC. November 2008. www. cdc. gov

Disinfection and Sterilization WA Rutala, DJ Weber, and HICPAC, www. cdc. gov EH Spaulding believed that how an object will be disinfected depended on the object’s intended use. CRITICAL - objects which enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (high-level disinfection [HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL -objects that touch only intact skin


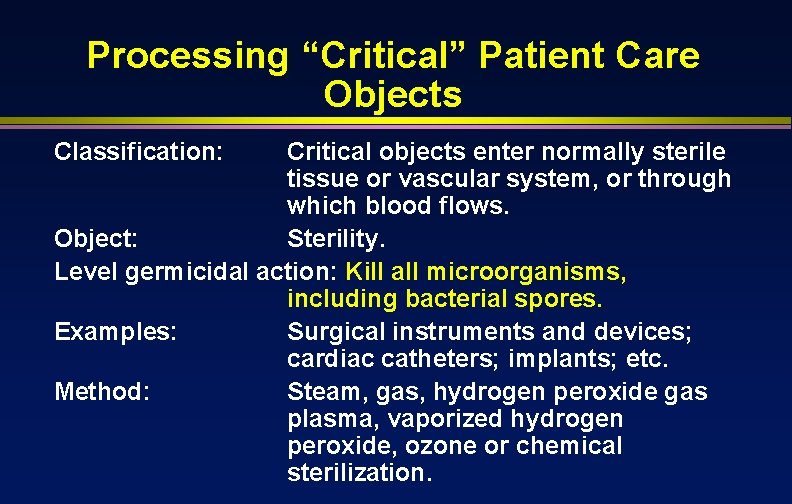
Processing “Critical” Patient Care Objects Classification: Critical objects enter normally sterile tissue or vascular system, or through which blood flows. Object: Sterility. Level germicidal action: Kill all microorganisms, including bacterial spores. Examples: Surgical instruments and devices; cardiac catheters; implants; etc. Method: Steam, gas, hydrogen peroxide gas plasma, vaporized hydrogen peroxide, ozone or chemical sterilization.

Sterilization of “Critical Objects” Steam sterilization Hydrogen peroxide gas plasma Ethylene oxide Ozone Vaporized hydrogen peroxide Steam formaldehyde

Ozone and Hydrogen Peroxide • Sterizone VP 4, 510(k) FDA clearance, TSO 3 • • Canada Sterilizer has a 4. 4 ft 3 chamber Advantages/Disadvantages-not yet known

FDA Panel, May 2015, Recommended Sterilization of Duodenoscopes

Disinfection and Sterilization WA Rutala, DJ Weber, and HICPAC, www. cdc. gov EH Spaulding believed that how an object will be disinfected depended on the object’s intended use. CRITICAL - objects which enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (high-level disinfection [HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL -objects that touch only intact skin

Disinfection and Sterilization WA Rutala, DJ Weber, and HICPAC, www. cdc. gov EH Spaulding believed that how an object will be disinfected depended on the object’s intended use (modified). CRITICAL - objects which directly or secondarily (i. e. , via a mucous membrane such as duodenoscopes) enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (high-level disinfection [HLD]) that kills all microorganisms but high numbers of bacterial spores.


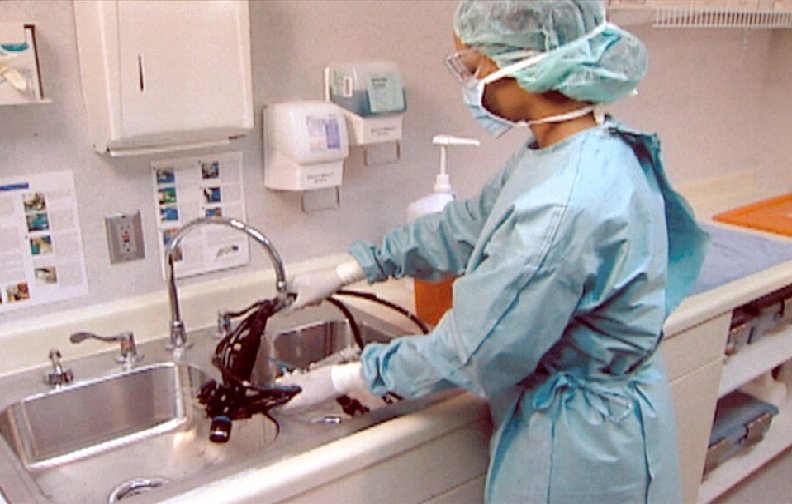
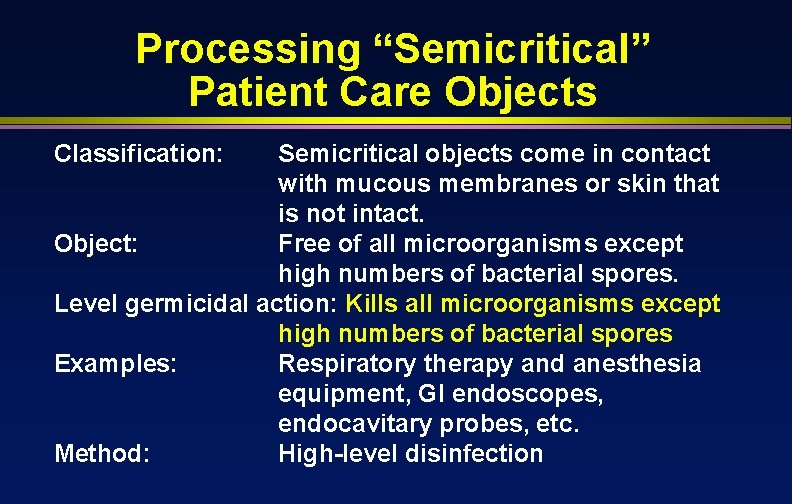
Processing “Semicritical” Patient Care Objects Classification: Semicritical objects come in contact with mucous membranes or skin that is not intact. Object: Free of all microorganisms except high numbers of bacterial spores. Level germicidal action: Kills all microorganisms except high numbers of bacterial spores Examples: Respiratory therapy and anesthesia equipment, GI endoscopes, endocavitary probes, etc. Method: High-level disinfection

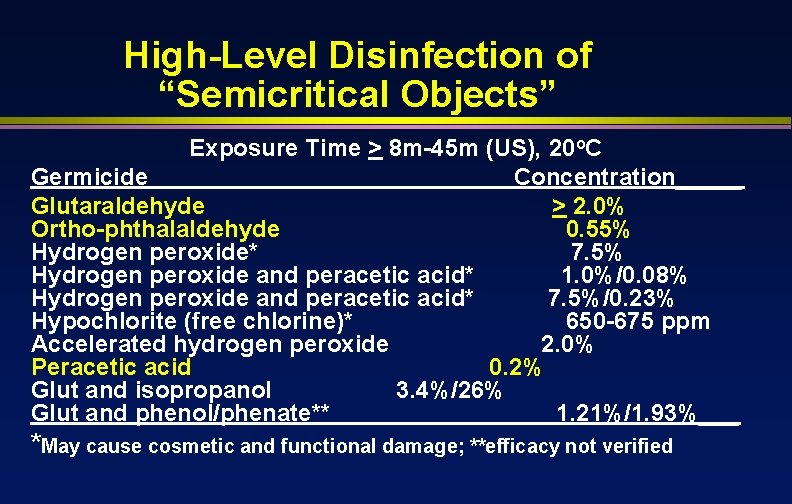
High-Level Disinfection of “Semicritical Objects” Exposure Time > 8 m-45 m (US), 20 o. C Germicide Concentration_____ Glutaraldehyde > 2. 0% Ortho-phthalaldehyde 0. 55% Hydrogen peroxide* 7. 5% Hydrogen peroxide and peracetic acid* 1. 0%/0. 08% Hydrogen peroxide and peracetic acid* 7. 5%/0. 23% Hypochlorite (free chlorine)* 650 -675 ppm Accelerated hydrogen peroxide 2. 0% Peracetic acid 0. 2% Glut and isopropanol 3. 4%/26% Glut and phenol/phenate** 1. 21%/1. 93%___ *May cause cosmetic and functional damage; **efficacy not verified


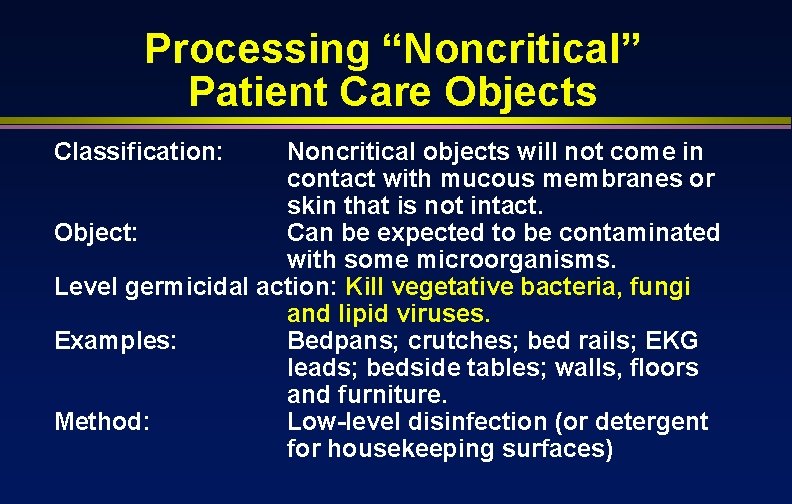
Processing “Noncritical” Patient Care Objects Classification: Noncritical objects will not come in contact with mucous membranes or skin that is not intact. Object: Can be expected to be contaminated with some microorganisms. Level germicidal action: Kill vegetative bacteria, fungi and lipid viruses. Examples: Bedpans; crutches; bed rails; EKG leads; bedside tables; walls, floors and furniture. Method: Low-level disinfection (or detergent for housekeeping surfaces)

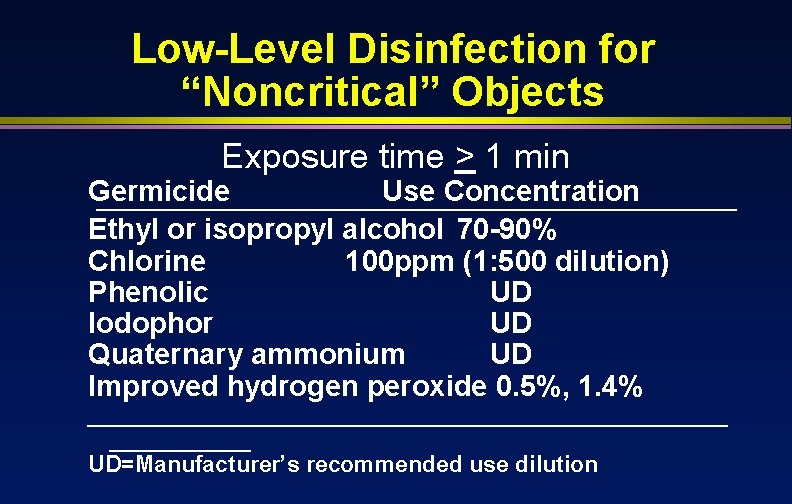
Low-Level Disinfection for “Noncritical” Objects Exposure time > 1 min Germicide Use Concentration Ethyl or isopropyl alcohol 70 -90% Chlorine 100 ppm (1: 500 dilution) Phenolic UD Iodophor UD Quaternary ammonium UD Improved hydrogen peroxide 0. 5%, 1. 4% _________________________ UD=Manufacturer’s recommended use dilution

Methods in Sterilization

Cleaning • Items must be cleaned using water with • • detergents or enzymatic cleaners before processing. Cleaning reduces the bioburden and removes foreign material (organic residue and inorganic salts) that interferes with the sterilization process. Cleaning and decontamination should be done as soon as possible after the items have been

Cleaning • Mechanical cleaning machines-automated equipment may increase productivity, improve cleaning effectiveness, and decrease worker exposure n n • n Utensil washer-sanitizer Ultrasonic cleaner Washer sterilizer Dishwasher Washer disinfector Manual


How Clean Is Clean? • • AAMI and FDA trying to gain consensus Reached consensus on maximum levels of top three common markers after a device is cleaned n Less than 6. 4 µg/cm 2 for protein n Less than 12 µg/cm 2 for total organic compound n Less than 2. 2 µg/cm 2 for hemoglobin Research needs to be performed to determine how healthcare facilities should verify cleanliness (real-time tests and meaningful analytical endpoints) Manufacturers’ ensure the HCF can clean the device (time, resources, device design)

Disinfection Practices

Disinfection and Sterilization EH Spaulding believed that how an object will be disinfected depended on the object’s intended use. CRITICAL - objects which enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (high-level disinfection [HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL -objects that touch only intact skin

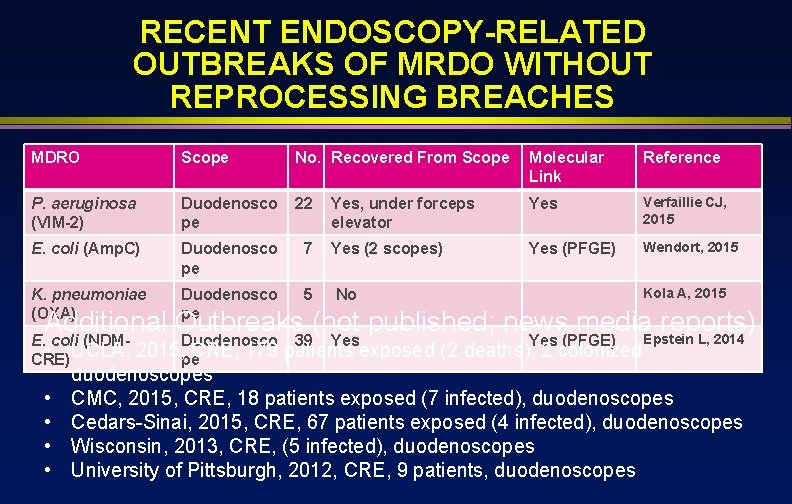
RECENT ENDOSCOPY-RELATED OUTBREAKS OF MRDO WITHOUT REPROCESSING BREACHES MDRO Scope No. Recovered From Scope Molecular Link Reference P. aeruginosa (VIM-2) Duodenosco pe 22 Yes, under forceps elevator Yes Verfaillie CJ, 2015 E. coli (Amp. C) Duodenosco pe 7 Yes (2 scopes) Yes (PFGE) Wendort, 2015 K. pneumoniae (OXA) Duodenosco pe 5 No E. coli (NDM • UCLA, CRE) Duodenosco 39 Kola A, 2015 Additional Outbreaks (not published; news media. Epstein reports) L, 2014 • • Yes (PFGE) 2015, pe. CRE, 179 patients exposed (2 deaths), 2 colonized duodenoscopes CMC, 2015, CRE, 18 patients exposed (7 infected), duodenoscopes Cedars-Sinai, 2015, CRE, 67 patients exposed (4 infected), duodenoscopes Wisconsin, 2013, CRE, (5 infected), duodenoscopes University of Pittsburgh, 2012, CRE, 9 patients, duodenoscopes

ENDOSCOPE REPROCESSING: CHALLENGES NDM-Producing E. coli Associated ERCP MMWR 2014; 62: 1051; Epstein et al. JAMA 2014; 312: 1447 -1455 NDM-producing E. coli recovered from elevator channel (elevator channel orients catheters, guide wires and accessories into the endoscope visual field; crevices difficult to access with cleaning brush and may impede effective reprocessing)

Reprocessing Channeled Endoscopes Cystoscopes, Ureteroscopes, Hysteroscopes


ENVIRONMENTAL CONTAMINATION LEADS TO HAIs • There is increasing evidence to support the • contribution of the environment to disease transmission This supports comprehensive disinfecting regimens (goal is not sterilization) to reduce the risk of acquiring a pathogen from the healthcare environment/equipment

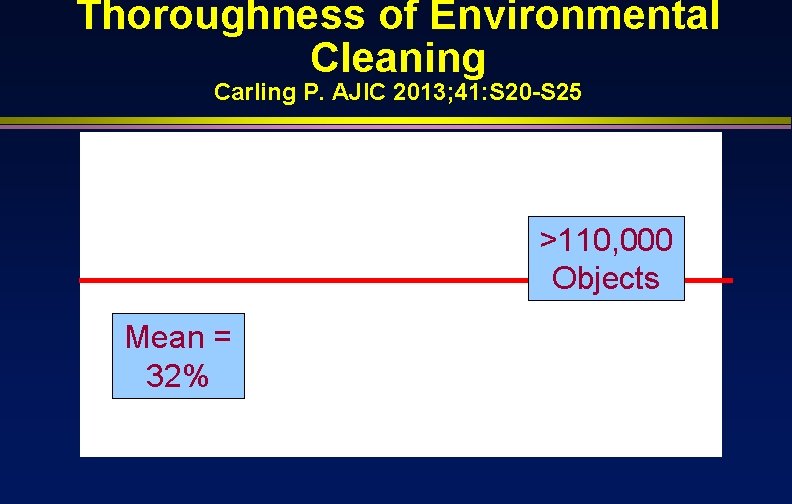
Thoroughness of Environmental Cleaning Carling P. AJIC 2013; 41: S 20 -S 25 >110, 000 Objects Mean = 32%

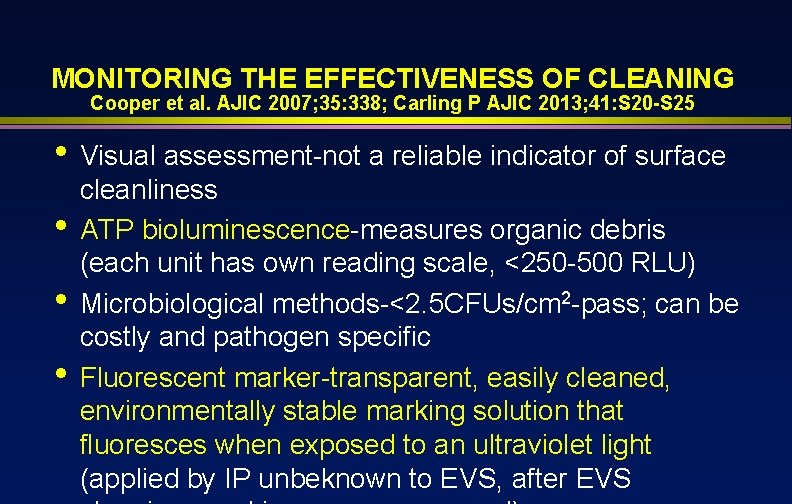
MONITORING THE EFFECTIVENESS OF CLEANING Cooper et al. AJIC 2007; 35: 338; Carling P AJIC 2013; 41: S 20 -S 25 • • Visual assessment-not a reliable indicator of surface cleanliness ATP bioluminescence-measures organic debris (each unit has own reading scale, <250 -500 RLU) Microbiological methods-<2. 5 CFUs/cm 2 -pass; can be costly and pathogen specific Fluorescent marker-transparent, easily cleaned, environmentally stable marking solution that fluoresces when exposed to an ultraviolet light (applied by IP unbeknown to EVS, after EVS

NEW “NO TOUCH” APPROACHES TO ROOM DECONTAMINATION Supplement Surface Disinfection Rutala, Weber. Infect Control Hosp Epidemiol. 2011; 32: 743

This technology should be considered for terminal room disinfection (e. g. , after discharge of patients under CP, during outbreaks) if studies continue to demonstrate a benefit.

Norovirus, C. difficile spores, MERS-Co. V, Enterovirus D 68, Ebola, MDR organisms such carbapenemase-producing Enterobacteriaceae (CRE), HPV, avian influenza A (H 7 N 9)

Antisepsis

Antiseptic Agents (used alone or in combination) • Alcohols, 60 -95% • Chlorhexidine, 2% and 4% aqueous • Iodophors • PCMX • Triclosan

Antiseptics • Hand Hygiene-improvement and compliance • • • monitoring Preoperative showers Preoperative skin preparation Surgical hand scrub Skin preparation prior to insertion of catheters Routine daily bathing of patients

Disinfection, Sterilization and Antisepsis • Provide overview of disinfection, sterilization and antisepsis n Indications and methods for sterilization, high-level disinfection and low-level disinfection n Cleaning of patient-care devices n Sterilization n Disinfection (high-level and low-level disinfection)

Summary D/S evidenced-based recommendations must be followed to prevent exposure to pathogens that may lead to infection Antiseptics must be used optimally to prevent infections that originate from the skin and patient contact

THANK YOU! www. disinfectionandsterilization. o rg