DiseaseSpecific Methods and Strategies for Monitoring Relapse Following































- Slides: 77

Disease-Specific Methods and Strategies for Monitoring Relapse Following Allogeneic Stem Cell Transplantation Pediatric Acute Lymphoblastic Leukemia Peter Bader, Wendy Stock, Andre Willasch, Alan Wayne on behalf of the Sub-Committee

Surveillance of Remission n Two principle approaches: n Chimerism n n Characterization of post transplant hematopoiesis MRD n Direct detection of the underlying malignancy

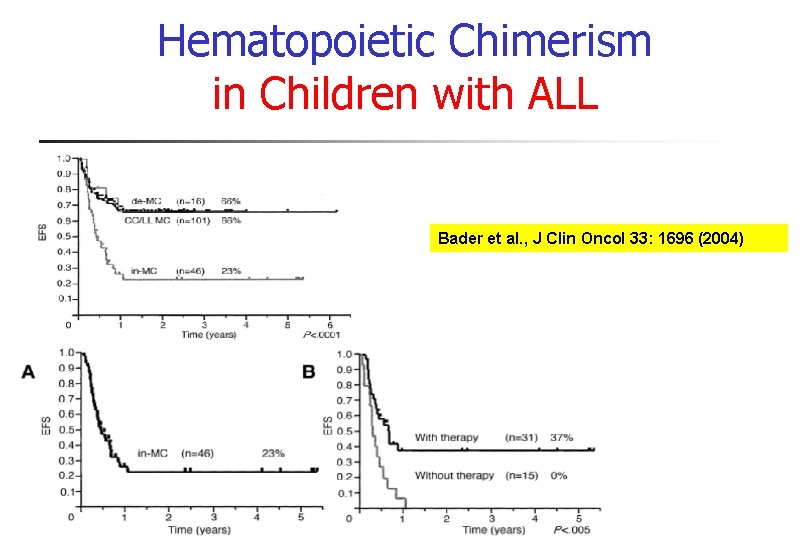
Hematopoietic Chimerism in Children with ALL Bader et al. , J Clin Oncol 33: 1696 (2004)

Studies on Chimerism and Intervention Author Formakova Haematologica 2003 Gorczynska BMT 2004 Bader JCO 2004 Horn BMT 2008 Number of patients Diagnosis Interval of 54 AL, CML and MDS children Methods Relapses weekly to +100; monthly STR MC associated with rejection and relapse Immunotherapy was possible 14 ALL, AML children weekly to +100; monthly STR In-MC could be converted by immunotherapy to CC 163 ALL children weekly to +100; monthly STR MC associated with rejection and relapse Immunotherapy was possible AL children 1, 3, 6, 12 months; In MC biweekly STR MC associated with relapse IT was not possible 20 investigations

Conclusions I n n Immunotherapy (WD of immunosuppression, DLI) is principally effective as pre-emptive treatment Chimerism can be used as surrogate marker for identifying patients at risk for impending relapse n However: n Not in all patients! Additional role for n MRD?

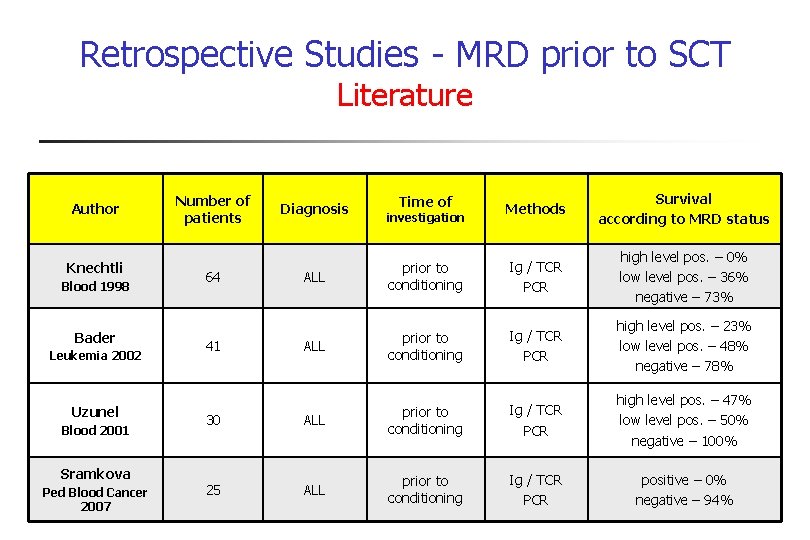
Retrospective Studies - MRD prior to SCT Literature Author Knechtli Blood 1998 Bader Leukemia 2002 Uzunel Blood 2001 Sramkova Ped Blood Cancer 2007 Methods Survival according to MRD status ALL prior to conditioning Ig / TCR PCR high level pos. – 0% low level pos. – 36% negative – 73% ALL prior to conditioning Ig / TCR PCR high level pos. – 23% low level pos. – 48% negative – 78% 30 ALL prior to conditioning Ig / TCR PCR high level pos. – 47% low level pos. – 50% negative – 100% 25 ALL prior to conditioning Ig / TCR PCR positive – 0% negative – 94% Number of patients 64 41 Diagnosis Time of investigation

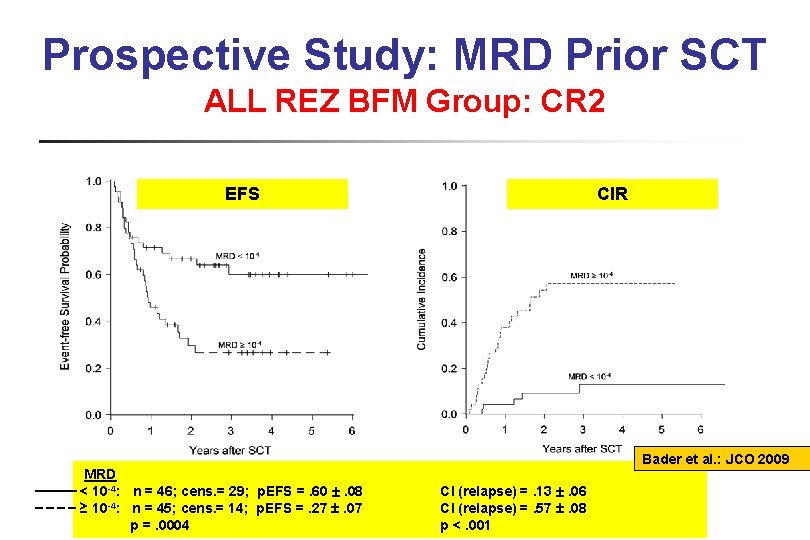
Prospective Study: MRD Prior SCT ALL REZ BFM Group: CR 2 EFS CIR CI Bader et al. : JCO 2009 MRD < 10 -4: n = 46; cens. = 29; p. EFS =. 60 . 08 ≥ 10 -4: n = 45; cens. = 14; p. EFS =. 27 . 07 p =. 0004 CI (relapse) =. 13 . 06 CI (relapse) =. 57 . 08 p <. 001

Conclusions II n n MRD prior to stem cell transplantation has a profound impact on post transplant outcome! What adds MRD post transplant?

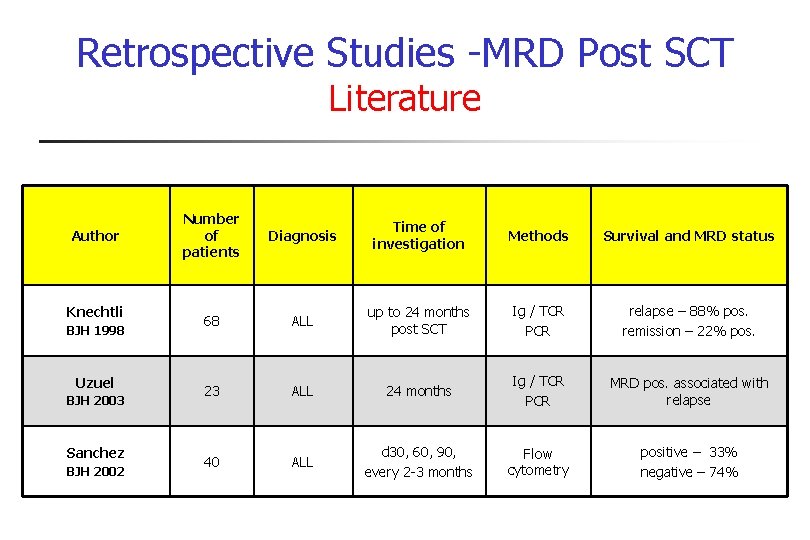
Retrospective Studies -MRD Post SCT Literature Author Knechtli BJH 1998 Uzuel BJH 2003 Sanchez BJH 2002 Number of patients Diagnosis Time of investigation Methods Survival and MRD status 68 ALL up to 24 months post SCT Ig / TCR PCR relapse – 88% pos. remission – 22% pos. 23 ALL 24 months Ig / TCR PCR MRD pos. associated with relapse 40 ALL d 30, 60, 90, every 2 -3 months Flow cytometry positive – 33% negative – 74%

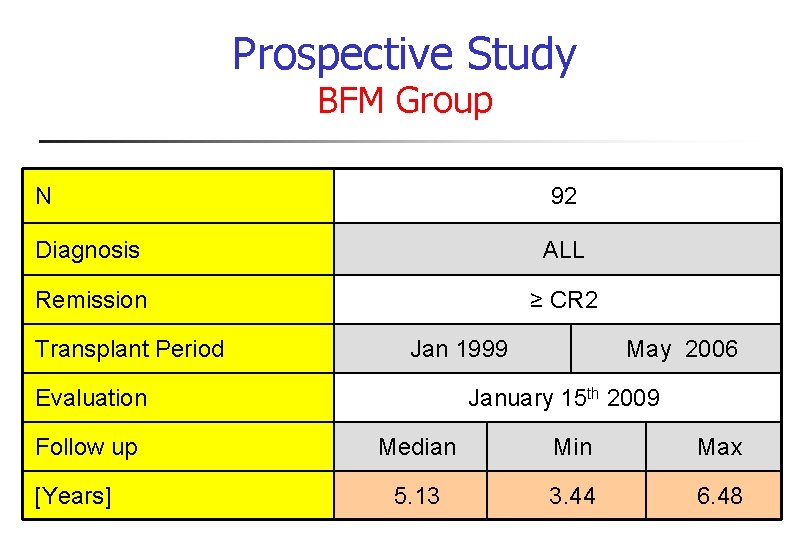
Prospective Study BFM Group N 92 Diagnosis ALL Remission ≥ CR 2 Transplant Period Jan 1999 Evaluation Follow up [Years] May 2006 January 15 th 2009 Median Min Max 5. 13 3. 44 6. 48

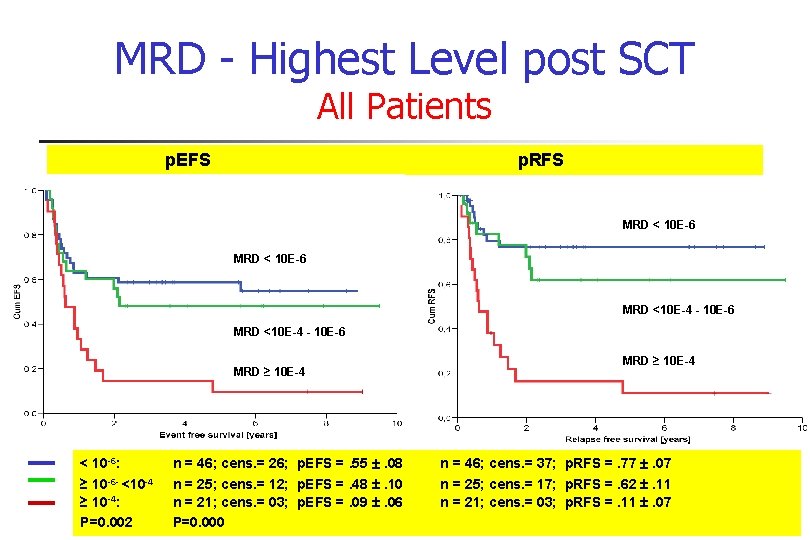
MRD - Highest Level post SCT All Patients p. EFS p. RFS MRD < 10 E-6 MRD <10 E-4 - 10 E-6 MRD ≥ 10 E-4 < 10 -6: ≥ 10 -6 - <10 -4 ≥ 10 -4: P=0. 002 MRD ≥ 10 E-4 n = 46; cens. = 26; p. EFS =. 55 . 08 n = 46; cens. = 37; p. RFS =. 77 . 07 n = 25; cens. = 12; p. EFS =. 48 . 10 n = 25; cens. = 17; p. RFS =. 62 . 11 n = 21; cens. = 03; p. EFS =. 09 . 06 n = 21; cens. = 03; p. RFS =. 11 . 07 P=0. 000

Conclusions III and Summary n MRD assessment in BM post transplant is predictive for relapse n n n Serial BM investigations are warranted. Current working recommendations of the BFM: days 30, 60, 100, 200, 365, at 18 months and 24 months. Summary: n n Patients with mixed chimerism have a high risk for relapse Patients, who become/remain MRD positive >10 -4, have a very high risk to develop relapse n Additional treatment in these patients is warranted

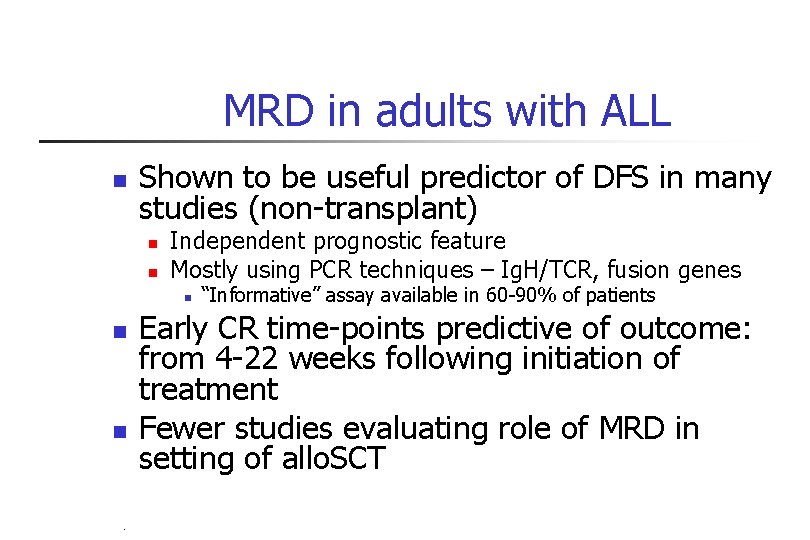
MRD in adults with ALL n Shown to be useful predictor of DFS in many studies (non-transplant) n n Independent prognostic feature Mostly using PCR techniques – Ig. H/TCR, fusion genes n n n “Informative” assay available in 60 -90% of patients Early CR time-points predictive of outcome: from 4 -22 weeks following initiation of treatment Fewer studies evaluating role of MRD in setting of allo. SCT PB-04/06 tk 05. 06

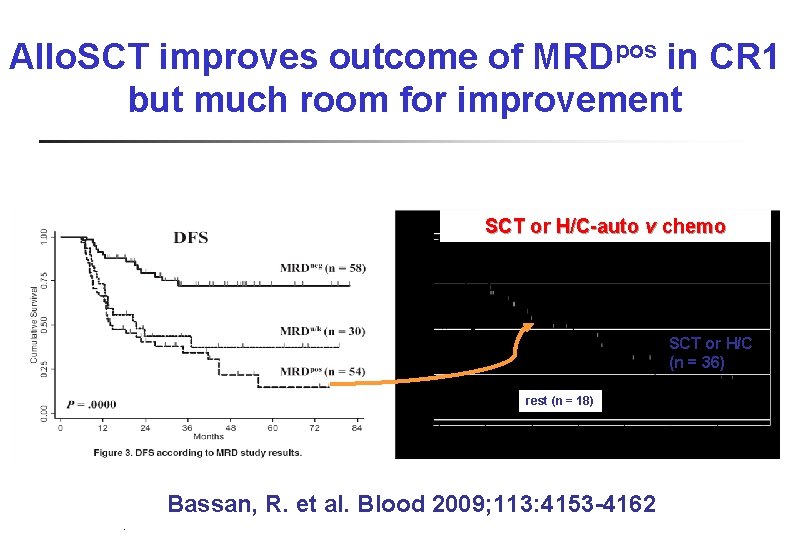
Allo. SCT improves outcome of MRDpos in CR 1 but much room for improvement SCT or H/C-auto v chemo SCT or H/C (n = 36) rest (n = 18) Bassan, R. et al. Blood 2009; 113: 4153 -4162 PB-04/06 tk 05. 06

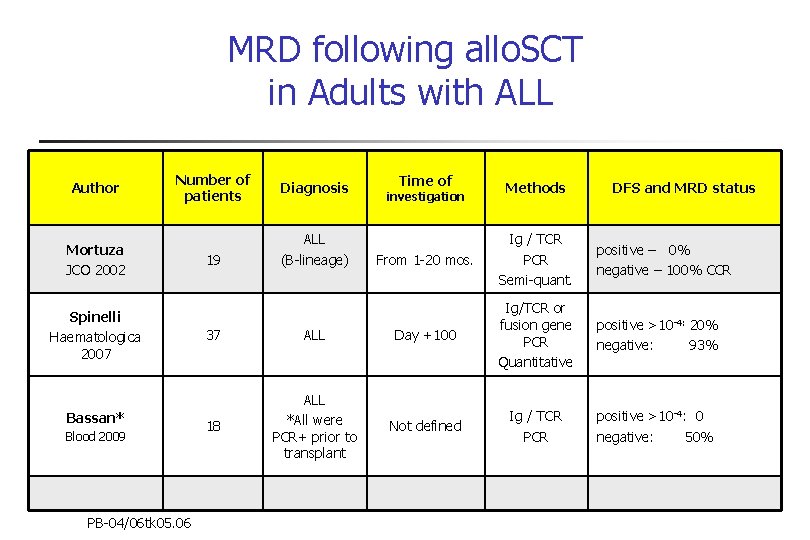
MRD following allo. SCT in Adults with ALL Author Number of patients Mortuza JCO 2002 Spinelli Haematologica 2007 Bassan* Blood 2009 PB-04/06 tk 05. 06 19 Diagnosis ALL (B-lineage) Time of investigation Methods DFS and MRD status From 1 -20 mos. Ig / TCR PCR Semi-quant. positive – 0% negative – 100% CCR positive >10 -4: 20% negative: 93% positive >10 -4: 0 negative: 50% 37 ALL Day +100 Ig/TCR or fusion gene PCR Quantitative 18 ALL *All were PCR+ prior to transplant Not defined Ig / TCR PCR

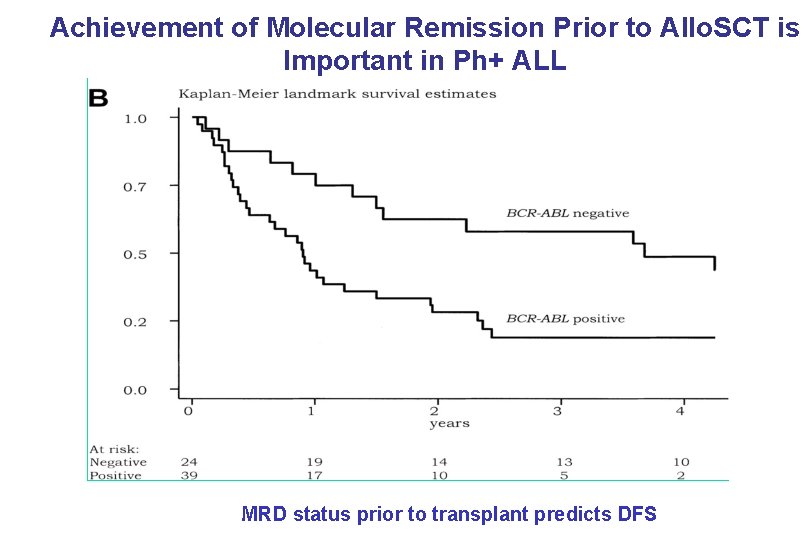
Achievement of Molecular Remission Prior to Allo. SCT is Important in Ph+ ALL Dombret et al: Blood MRD status prior to transplant predicts DFS PB-04/06 tk 05. 06 100: 2002

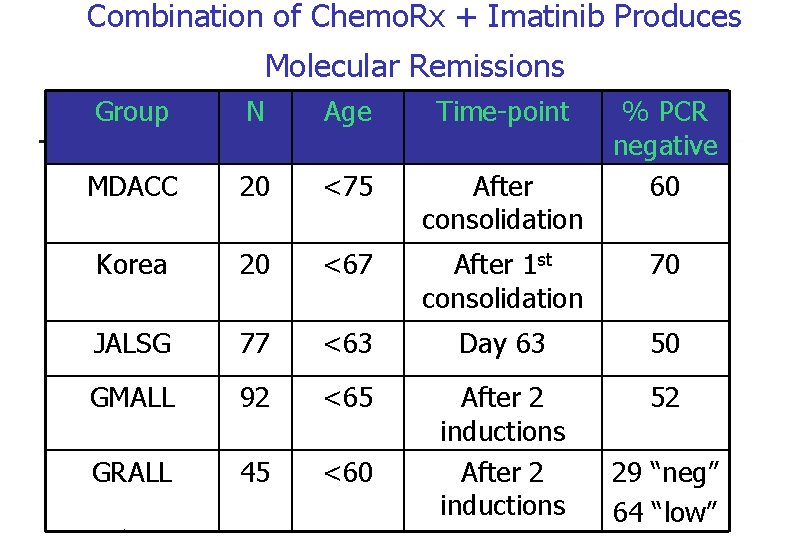
Combination of Chemo. Rx + Imatinib Produces Molecular Remissions Group N Age Time-point MDACC 20 <75 After consolidation Korea 20 <67 After 1 st consolidation 70 JALSG 77 <63 Day 63 50 GMALL 92 <65 52 GRALL 45 <60 After 2 inductions PB-04/06 tk 05. 06 % PCR negative 60 29 “neg” 64 “low”

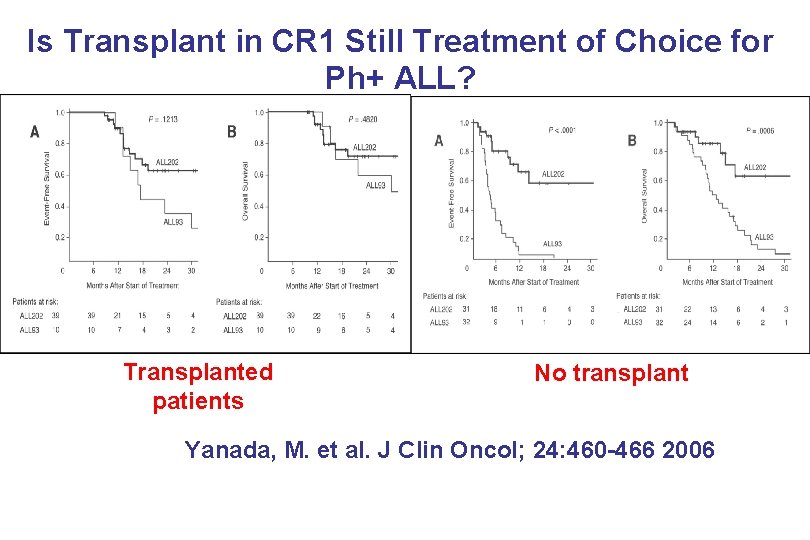
Is Transplant in CR 1 Still Treatment of Choice for Ph+ ALL? Transplanted patients No transplant Yanada, M. et al. J Clin Oncol; 24: 460 -466 2006 PB-04/06 tk 05. 06

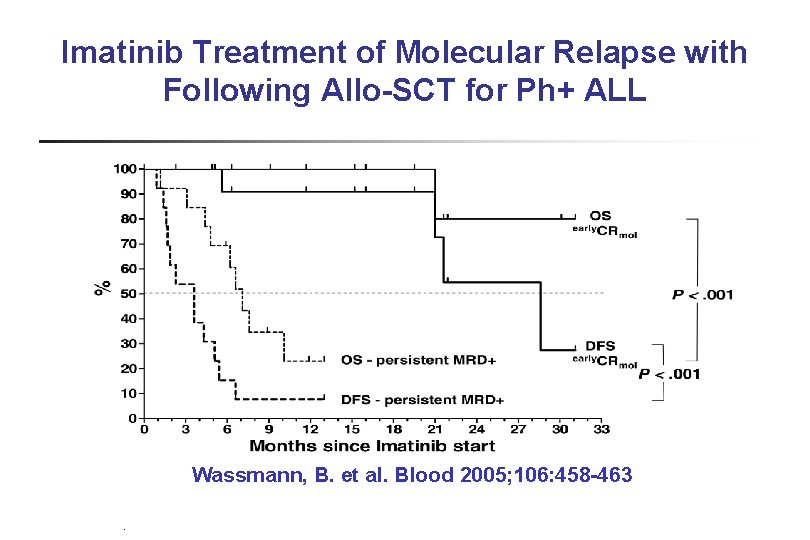
Imatinib Treatment of Molecular Relapse with Following Allo-SCT for Ph+ ALL Wassmann, B. et al. Blood 2005; 106: 458 -463 PB-04/06 tk 05. 06

Summary n MRD detection both prior to and following allo. SCT for adults with ALL is associated with poor DFS n Clinical interventions based on MRD measurements suggest utility but data are very limited: n n Allocation to allo. SCT in CR 1 Post-transplant intervention to prevent relapse n n Targeted therapy (e. g. imatinib) following transplant Challenge: implementation of standardized MRD assays that can be done in “real-time” n n Ig. H/TCR q. PCR assays are laborious Data on flow cytometric measurements of MRD in adults with ALL are lacking PB-04/06 tk 05. 06

Disease-Specific Methods and Strategies for Monitoring Relapse Following Allogeneic Stem Cell Transplantation Chronic Lymphocytic Leukemia Sebastian Böttcher, Issa Khouri, Peter Dreger

Overview • Techniques • MRD kinetics • Clinical significance of MRD

Techniques

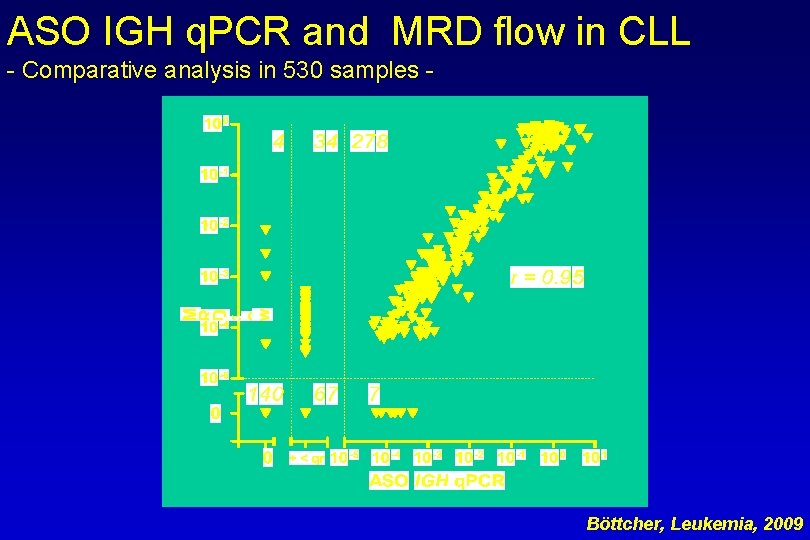
ASO IGH q. PCR and MRD flow in CLL - Comparative analysis in 530 samples - Böttcher, Leukemia, 2009

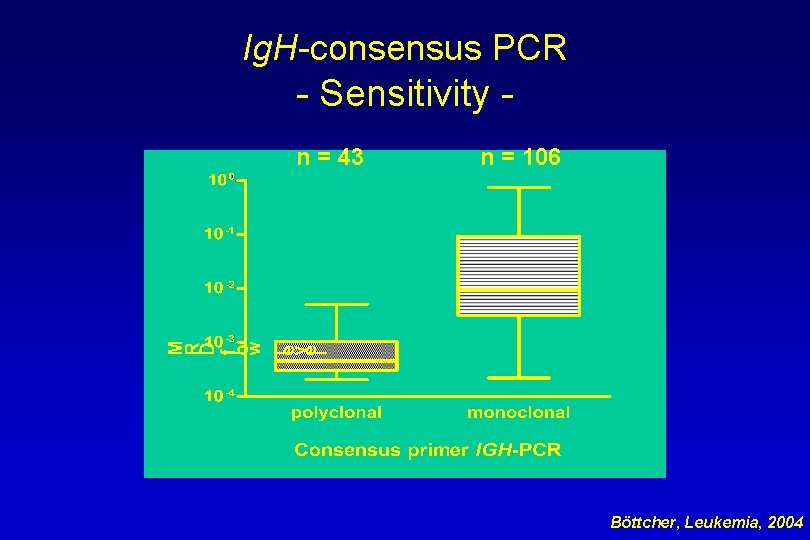
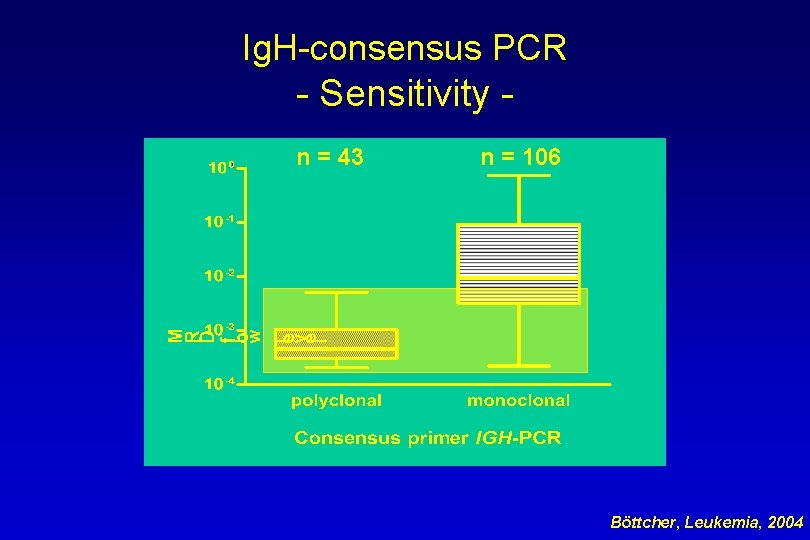
Ig. H-consensus PCR - Sensitivity n = 43 n = 106 Böttcher, Leukemia, 2004

Ig. H-consensus PCR - Sensitivity n = 43 n = 106 Böttcher, Leukemia, 2004

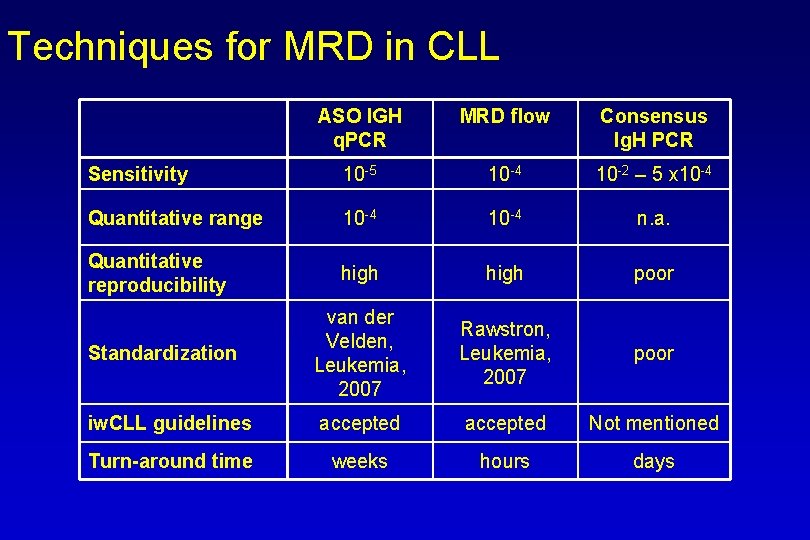
Techniques for MRD in CLL ASO IGH q. PCR MRD flow Consensus Ig. H PCR Sensitivity 10 -5 10 -4 10 -2 – 5 x 10 -4 Quantitative range 10 -4 n. a. Quantitative reproducibility high poor Standardization van der Velden, Leukemia, 2007 Rawstron, Leukemia, 2007 poor iw. CLL guidelines accepted Not mentioned Turn-around time weeks hours days

MRD kinetics

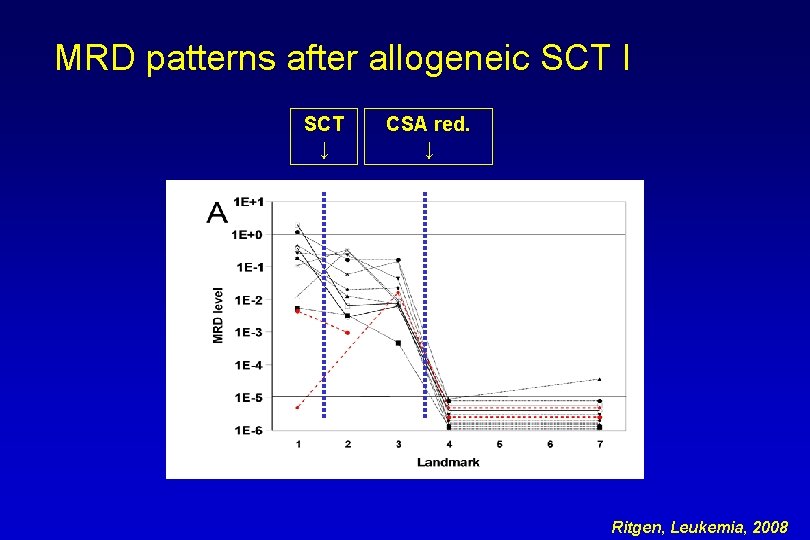
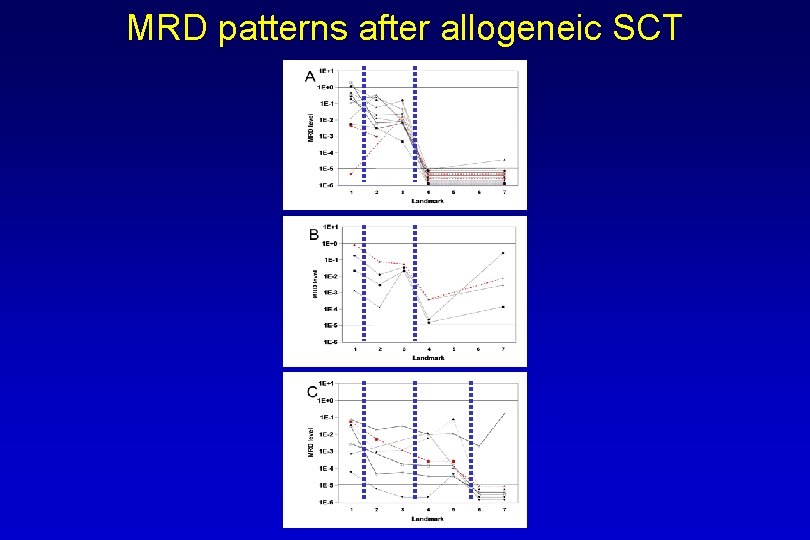
MRD patterns after allogeneic SCT I SCT ↓ CSA red. ↓ Ritgen, Leukemia, 2008

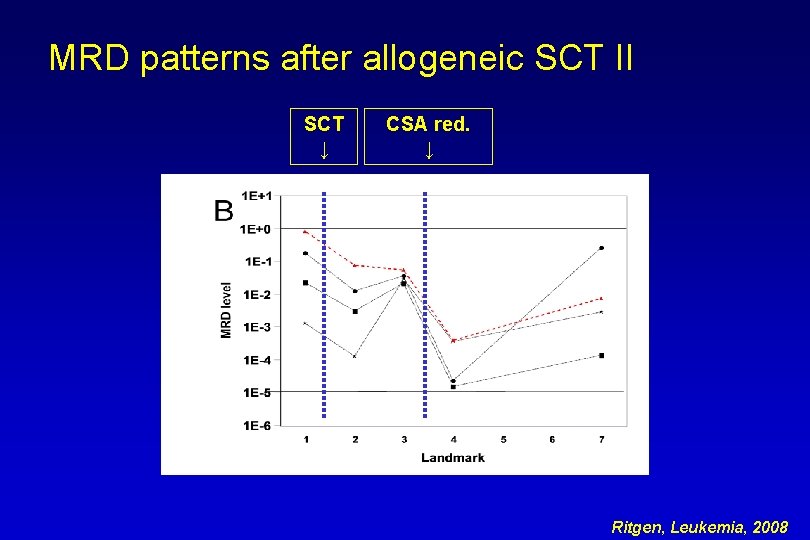
MRD patterns after allogeneic SCT II SCT ↓ CSA red. ↓ Ritgen, Leukemia, 2008

MRD patterns after allogeneic SCT III SCT ↓ CSA red. ↓ DLI ↓ Ritgen, Leukemia, 2008

MRD patterns after allogeneic SCT

Prognostic significance

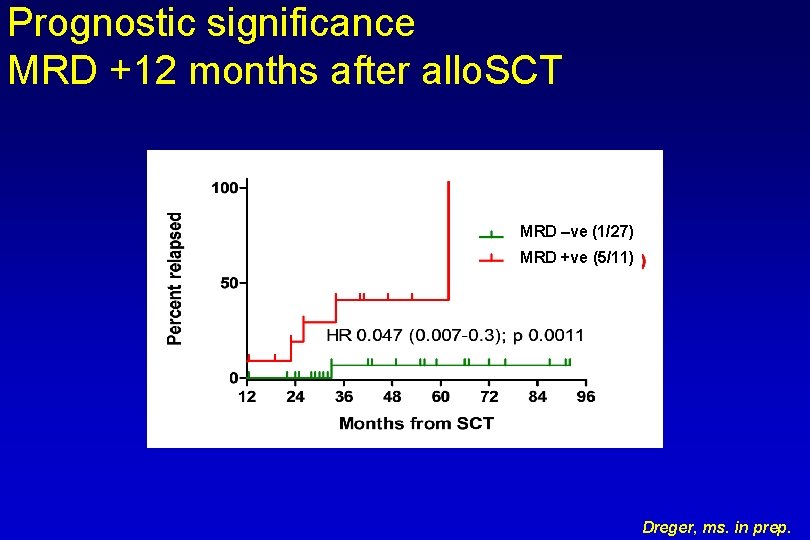
Prognostic significance MRD +12 months after allo. SCT MRD –ve (1/27) MRD +ve (5/11) Dreger, ms. in prep.

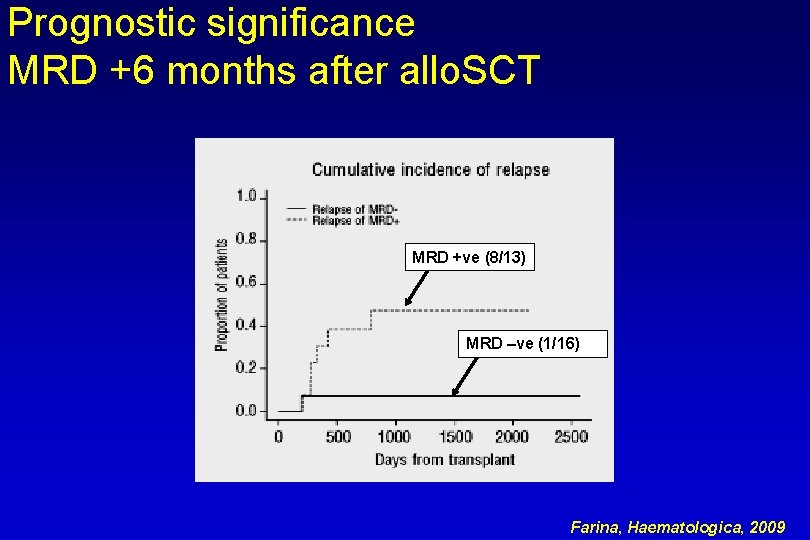
Prognostic significance MRD +6 months after allo. SCT MRD +ve (8/13) MRD –ve (1/16) Farina, Haematologica, 2009

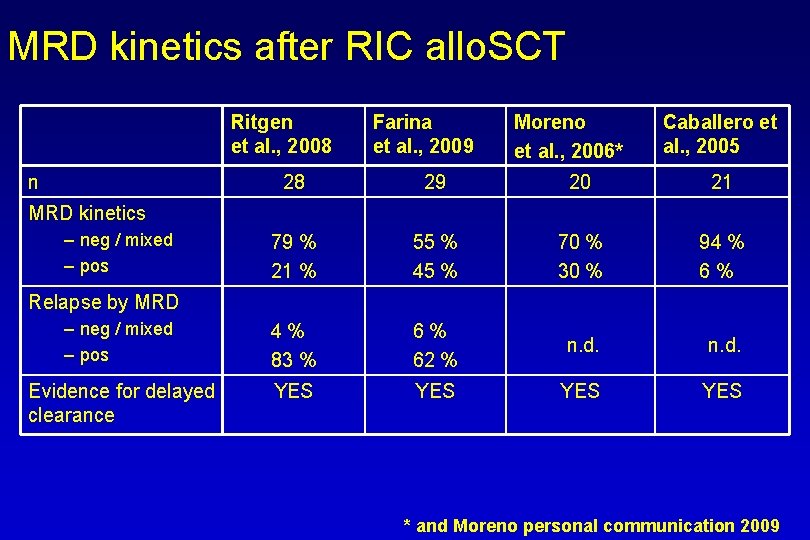
MRD kinetics after RIC allo. SCT Ritgen et al. , 2008 n Farina et al. , 2009 Moreno et al. , 2006* Caballero et al. , 2005 28 29 20 21 79 % 21 % 55 % 45 % 70 % 30 % 94 % 6% – neg / mixed – pos 4% 83 % 6% 62 % n. d. Evidence for delayed clearance YES YES MRD kinetics – neg / mixed – pos Relapse by MRD * and Moreno personal communication 2009

Summary: MRD after allo. SCT • Techniques: have to be quantitative & sensitive ( 10 -4) • MRD flow • ASO Ig. H q. PCR • Retrospective analyses show that: • delayed, likely GVL-mediated MRD clearance occurs • MRD clearance: • predicts of very low relapse risk • is durable • might serve as surrogate marker for cure • MRD persistence after Cs. A tapering can be used as trigger for preemptive immun-therapy (DLI) Ø Treatment aim to be tested prospectively : MRD negativity (< 10 -4) 12 months after allo. SCT

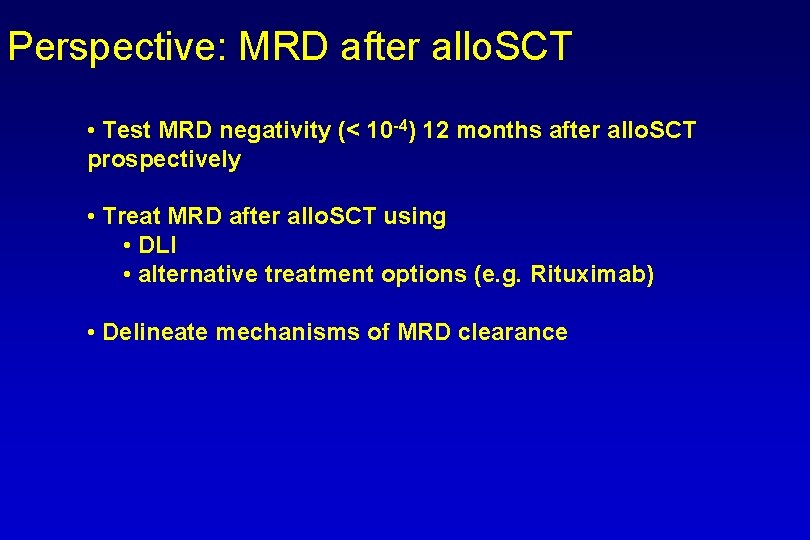
Perspective: MRD after allo. SCT • Test MRD negativity (< 10 -4) 12 months after allo. SCT prospectively • Treat MRD after allo. SCT using • DLI • alternative treatment options (e. g. Rituximab) • Delineate mechanisms of MRD clearance

Relapse Monitoring after Allogeneic Stem Cell Transplantation for Lymphomas Issa Khouri, Julie Vose

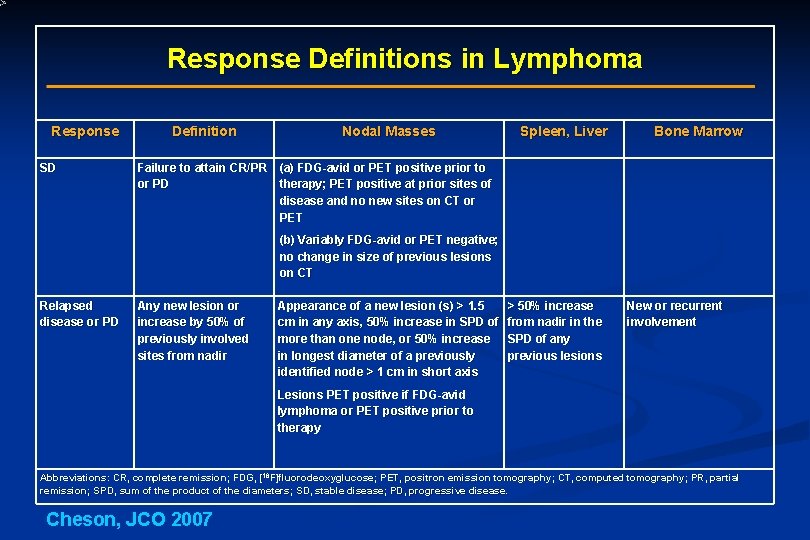
Response Definitions in Lymphoma Response SD Definition Nodal Masses Spleen, Liver Bone Marrow Failure to attain CR/PR (a) FDG-avid or PET positive prior to or PD therapy; PET positive at prior sites of disease and no new sites on CT or PET (b) Variably FDG-avid or PET negative; no change in size of previous lesions on CT Relapsed disease or PD Any new lesion or increase by 50% of previously involved sites from nadir Appearance of a new lesion (s) > 1. 5 cm in any axis, 50% increase in SPD of more than one node, or 50% increase in longest diameter of a previously identified node > 1 cm in short axis > 50% increase from nadir in the SPD of any previous lesions New or recurrent involvement Lesions PET positive if FDG-avid lymphoma or PET positive prior to therapy Abbreviations: CR, complete remission; FDG, [18 F]fluorodeoxyglucose; PET, positron emission tomography; CT, computed tomography; PR, partial remission; SPD, sum of the product of the diameters; SD, stable disease; PD, progressive disease. Cheson, JCO 2007

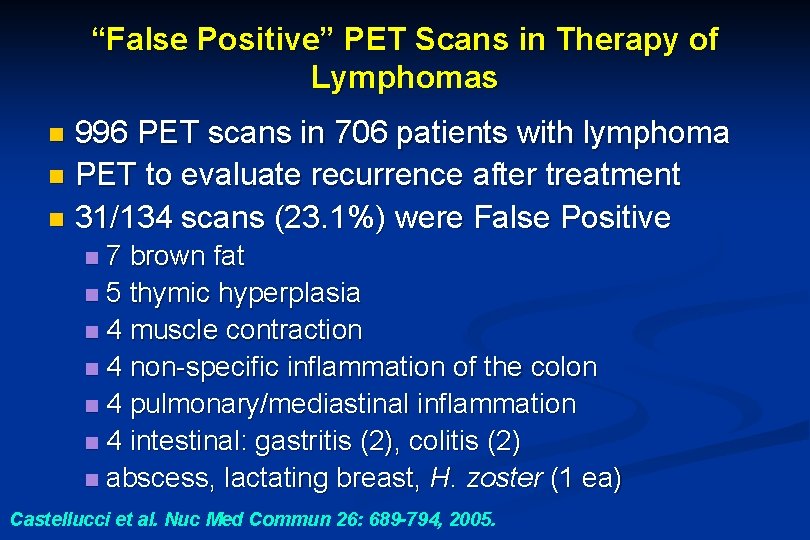
“False Positive” PET Scans in Therapy of Lymphomas 996 PET scans in 706 patients with lymphoma n PET to evaluate recurrence after treatment n 31/134 scans (23. 1%) were False Positive n 7 brown fat n 5 thymic hyperplasia n 4 muscle contraction n 4 non-specific inflammation of the colon n 4 pulmonary/mediastinal inflammation n 4 intestinal: gastritis (2), colitis (2) n abscess, lactating breast, H. zoster (1 ea) n Castellucci et al. Nuc Med Commun 26: 689 -794, 2005.

Examples of Bone Marrow Findings on PET in Two Patients with NHL BM Involvement Present No BM Involvement, GCSF(+) Message: The films look the same!

Survival in NHL Relapsing post Allo Using CT Criteria Khouri et al. unpublished data

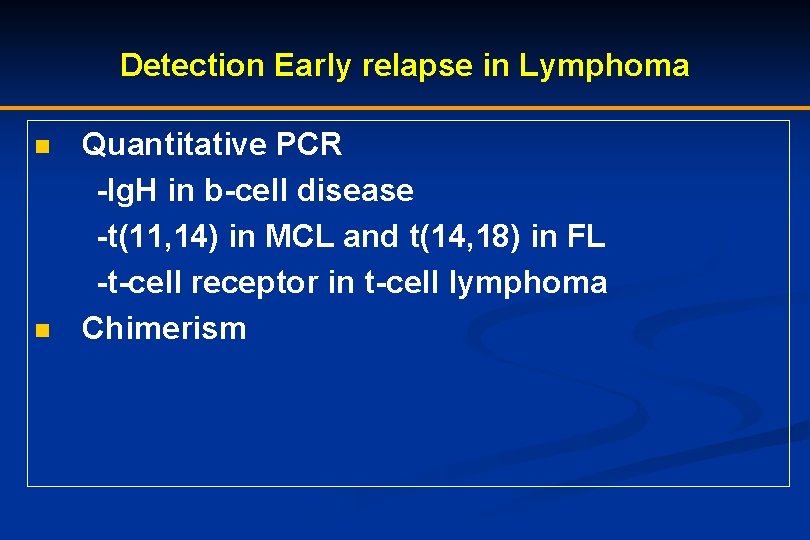
Detection Early relapse in Lymphoma Quantitative PCR -Ig. H in b-cell disease -t(11, 14) in MCL and t(14, 18) in FL -t-cell receptor in t-cell lymphoma n Chimerism n

Chimerism at day 90 and Outcome post NST Chimerism Mixed Full Donor 17 16 No. of patients CR: PR at NST, % 29 71 63 38 Achieved CR, no. (%) 17 (100) 16 (100) Chronic GVHD, no. (%) 10 (59) 11 (69) No. of relapse, (%) 1 (6) P value 0. 06 0. 5 Khouri, Blood 2008

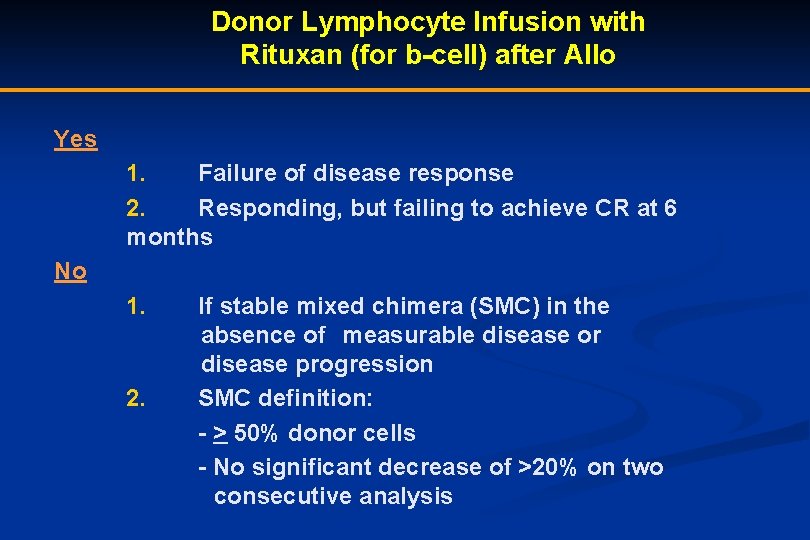
Donor Lymphocyte Infusion with Rituxan (for b-cell) after Allo Yes 1. Failure of disease response 2. Responding, but failing to achieve CR at 6 months No 1. If stable mixed chimera (SMC) in the absence of measurable disease or disease progression 2. SMC definition: - > 50% donor cells - No significant decrease of >20% on two consecutive analysis

Disease specific Monitoring of Relapse after Allogeneic Hematopoietic Cell Transplantation Multiple Myeloma NCI Workshop 1. /2. -11. 2009 Nicolaus Kröger

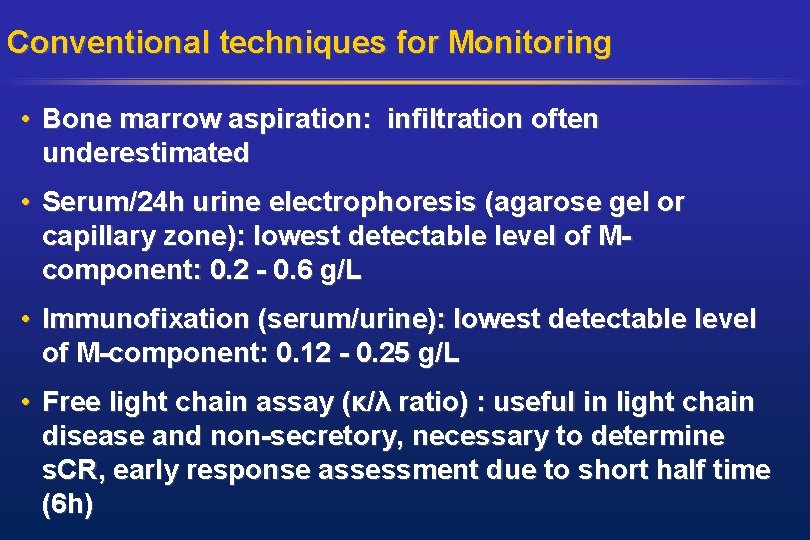
Conventional techniques for Monitoring • Bone marrow aspiration: infiltration often underestimated • Serum/24 h urine electrophoresis (agarose gel or capillary zone): lowest detectable level of Mcomponent: 0. 2 - 0. 6 g/L • Immunofixation (serum/urine): lowest detectable level of M-component: 0. 12 - 0. 25 g/L • Free light chain assay (κ/λ ratio) : useful in light chain disease and non-secretory, necessary to determine s. CR, early response assessment due to short half time (6 h)

Imaging monitoring • More than 80% of the pts develop osteolytic bone lesions • The hallmark of myeloma bone disease is an increased osteoclastic bone resorption and an exhausted osteoblast function resulting in a reduced bone formation even in patients in complete remission • Standard: conventional radiology as skeletal survey involving cervical, thoracic and lumbar spine, skull, chest, pelvis, humeri and femora • Disadvantage: low sensitivity, no exact response assessment • CT: high sensitivity, but higher radiation dose • MRI: high sensitivity, no radiation dose, detect extramedul-lary disease • PET-CT: highest sensitivity for extramedullary disease

Flow-cytometry • Flow cytometry has become an easy applicable method to detect residual myeloma cells The European Myeloma Network recommends a minimal panel including • CD 19, CD 56, CD 20, CD 117, CD 28 and CD 27. • Plasma cell gating should be based on CD 38 vs. CD 138 expression • This method is less sensitive (10 -4) than allele-specific oligonucleotides PCR (ASO-PCR) Rawstron 2008

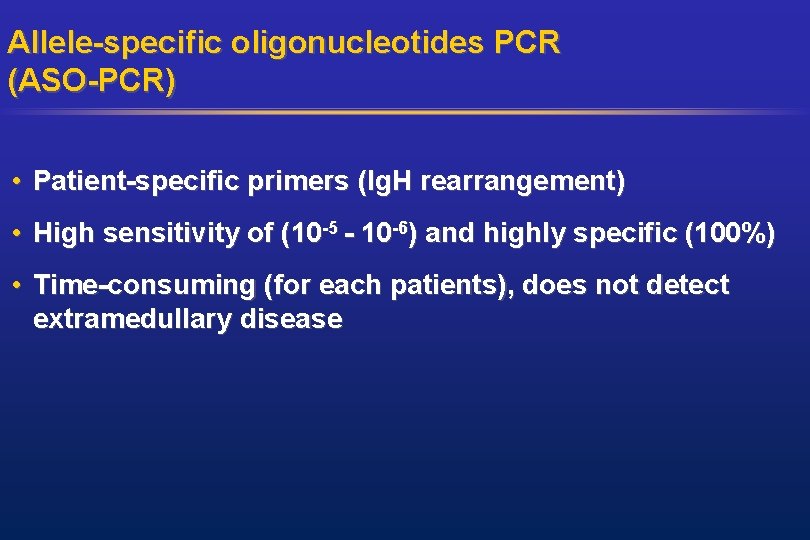
Allele-specific oligonucleotides PCR (ASO-PCR) • Patient-specific primers (Ig. H rearrangement) • High sensitivity of (10 -5 - 10 -6) and highly specific (100%) • Time-consuming (for each patients), does not detect extramedullary disease

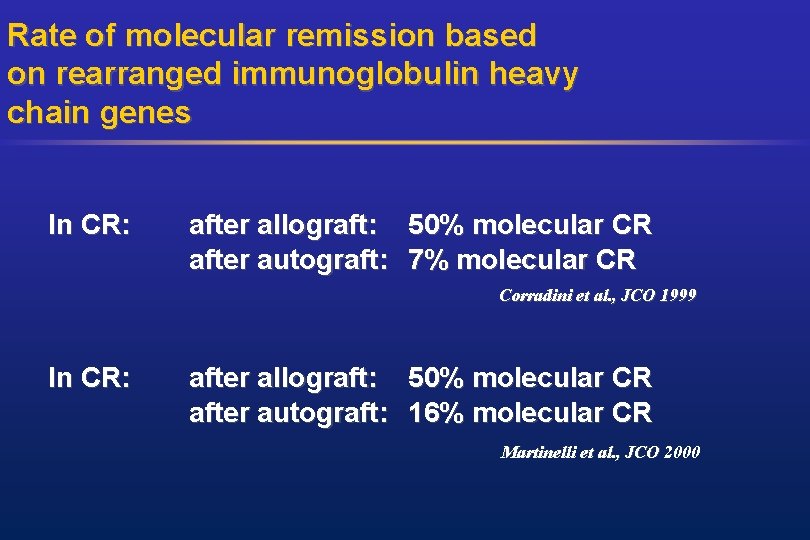
Rate of molecular remission based on rearranged immunoglobulin heavy chain genes In CR: after allograft: 50% molecular CR after autograft: 7% molecular CR Corradini et al. , JCO 1999 In CR: after allograft: 50% molecular CR after autograft: 16% molecular CR Martinelli et al. , JCO 2000

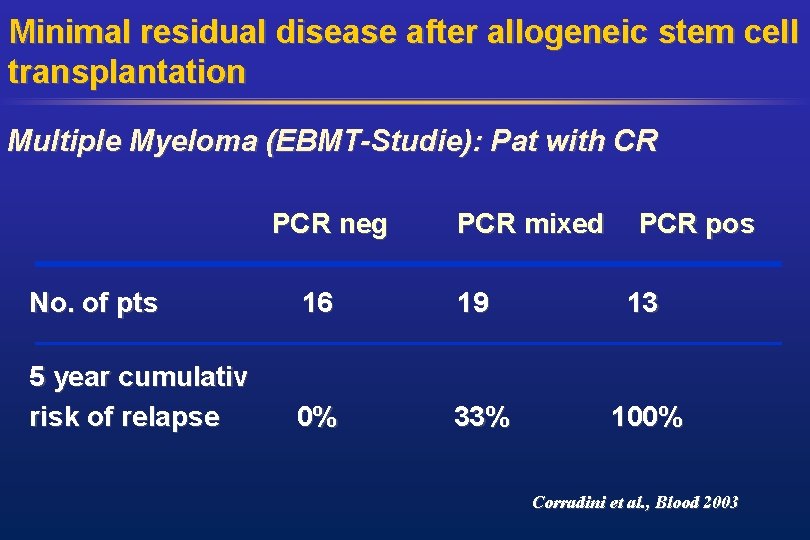
Minimal residual disease after allogeneic stem cell transplantation Multiple Myeloma (EBMT-Studie): Pat with CR PCR neg No. of pts 16 PCR mixed 19 PCR pos 13 5 year cumulativ risk of relapse 0% 33% 100% Corradini et al. , Blood 2003

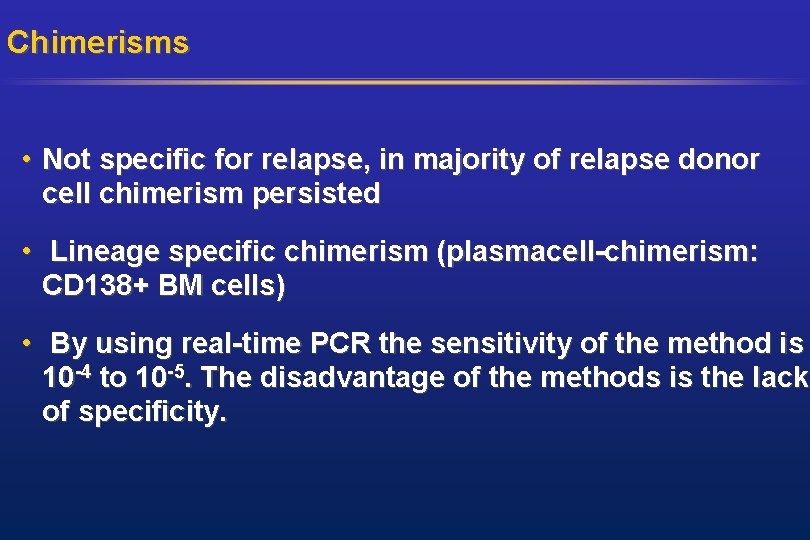
Chimerisms • Not specific for relapse, in majority of relapse donor cell chimerism persisted • Lineage specific chimerism (plasmacell-chimerism: CD 138+ BM cells) • By using real-time PCR the sensitivity of the method is 10 -4 to 10 -5. The disadvantage of the methods is the lack of specificity.

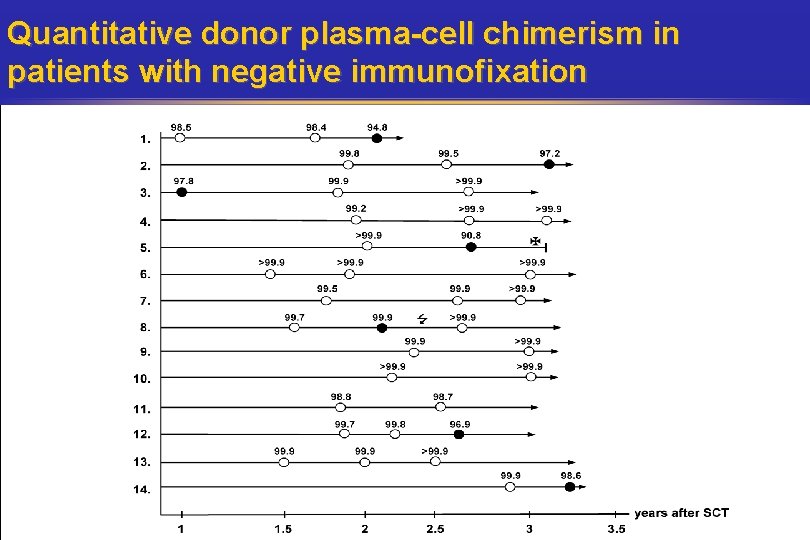
Quantitative donor plasma-cell chimerism in patients with negative immunofixation

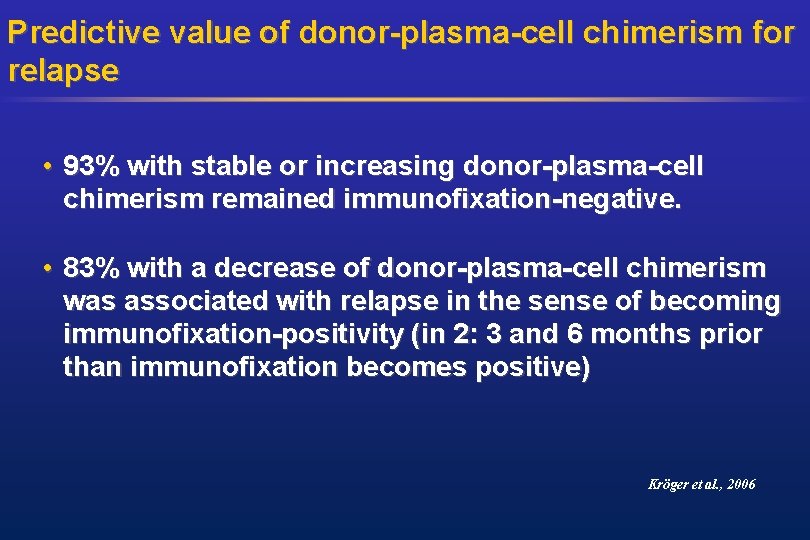
Predictive value of donor-plasma-cell chimerism for relapse • 93% with stable or increasing donor-plasma-cell chimerism remained immunofixation-negative. • 83% with a decrease of donor-plasma-cell chimerism was associated with relapse in the sense of becoming immunofixation-positivity (in 2: 3 and 6 months prior than immunofixation becomes positive) Kröger et al. , 2006

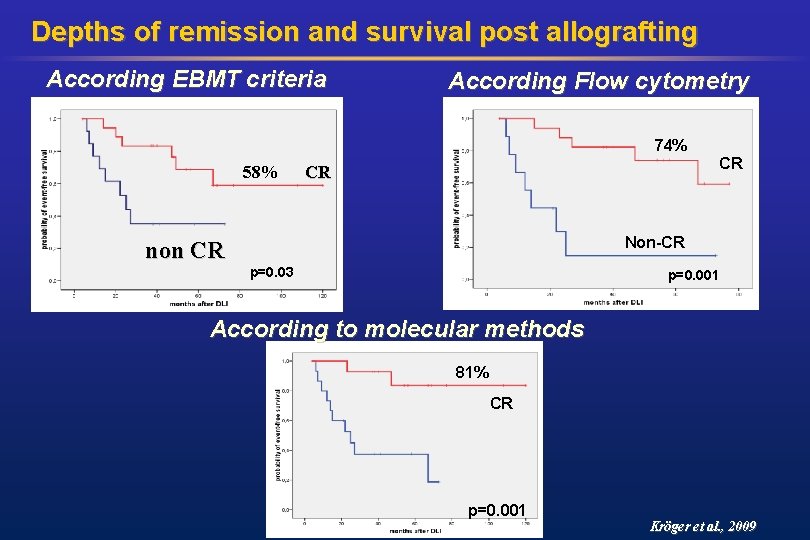
Depths of remission and survival post allografting According EBMT criteria According Flow cytometry 74% 58% CR CR Non-CR non CR p=0. 03 p=0. 001 According to molecular methods 81% CR p=0. 001 Kröger et al. , 2009

Relapse Definition NCI Workshop 1. /2. 11. 2009 Nicolaus Kröger Dept. of Stem Cell Transplantation, University Hospital Hamburg, Germany

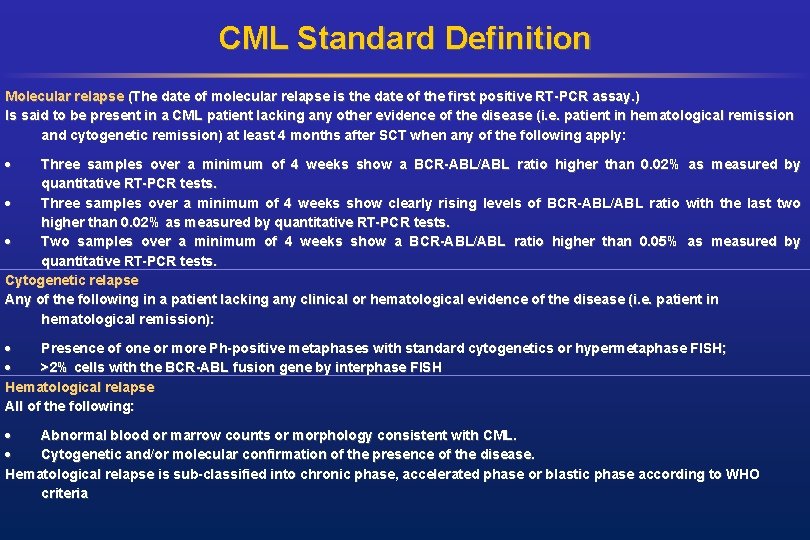
CML Standard Definition Molecular relapse (The date of molecular relapse is the date of the first positive RT-PCR assay. ) Is said to be present in a CML patient lacking any other evidence of the disease (i. e. patient in hematological remission and cytogenetic remission) at least 4 months after SCT when any of the following apply: Three samples over a minimum of 4 weeks show a BCR-ABL/ABL ratio higher than 0. 02% as measured by quantitative RT-PCR tests. Three samples over a minimum of 4 weeks show clearly rising levels of BCR-ABL/ABL ratio with the last two higher than 0. 02% as measured by quantitative RT-PCR tests. Two samples over a minimum of 4 weeks show a BCR-ABL/ABL ratio higher than 0. 05% as measured by quantitative RT-PCR tests. Cytogenetic relapse Any of the following in a patient lacking any clinical or hematological evidence of the disease (i. e. patient in hematological remission): Presence of one or more Ph-positive metaphases with standard cytogenetics or hypermetaphase FISH; >2% cells with the BCR-ABL fusion gene by interphase FISH Hematological relapse All of the following: Abnormal blood or marrow counts or morphology consistent with CML. Cytogenetic and/or molecular confirmation of the presence of the disease. Hematological relapse is sub-classified into chronic phase, accelerated phase or blastic phase according to WHO criteria

CML Proposal Disease Definition of CR Definition of Relapse Molecular marker Chromosome Chimerism CML Hematologic Cytogenetic Molecular BCR-ABL RT-PCR Cytogenetic (incl FISH ) PCR or VNTR/STR applicable All patients All patients q. PCR identifies relapse risk groups Not as sensitive as q. PCR for MRD detection Comment: Imaging Flow cytometry 4 -6 color flow Not applicable subgroups Only helpful in identifying aberrant blasts in advanced phase disease

Myelofibrosis Standard Definition Progressive Disease: Requires one of the following: • Progressive splenomegaly that is defined by the appearance of a previous absent splenomegaly that is palpable at greater than 5 cm below the left costal margin or a minimum of 100% increase in palpable distance for baseline splenomegaly of 5 -10 cm or a minimum of 50% increase in palpable distance for baseline splenomegaly of greater than 10 cm. • Leukemic transformation confirmed by bone marrow blast count of at least 20% • Increase in peripheral blood blast percentage of at least 20% that lasts for 8 weeks • Relapse: Changes from CR to PR or CR/PR to Clinical improvement

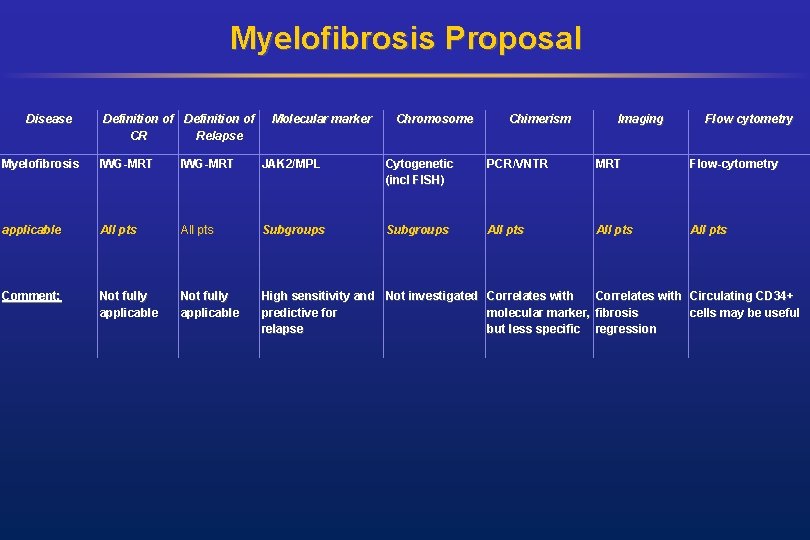
Myelofibrosis Proposal Disease Definition of CR Relapse Molecular marker Chromosome Chimerism Imaging Flow cytometry Myelofibrosis IWG-MRT JAK 2/MPL Cytogenetic (incl FISH) PCR/VNTR MRT Flow-cytometry applicable All pts Subgroups All pts Comment: Not fully applicable High sensitivity and Not investigated Correlates with Circulating CD 34+ predictive for molecular marker, fibrosis cells may be useful relapse but less specific regression

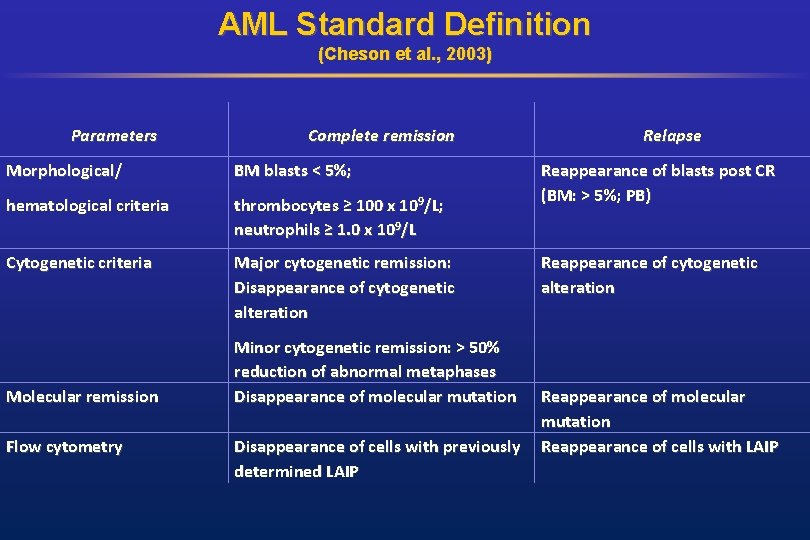
AML Standard Definition (Cheson et al. , 2003) Parameters Complete remission Morphological/ BM blasts < 5%; hematological criteria thrombocytes ≥ 100 x 109/L; neutrophils ≥ 1. 0 x 109/L Cytogenetic criteria Major cytogenetic remission: Disappearance of cytogenetic alteration Molecular remission Flow cytometry Minor cytogenetic remission: > 50% reduction of abnormal metaphases Disappearance of molecular mutation Disappearance of cells with previously determined LAIP Relapse Reappearance of blasts post CR (BM: > 5%; PB) Reappearance of cytogenetic alteration Reappearance of molecular mutation Reappearance of cells with LAIP

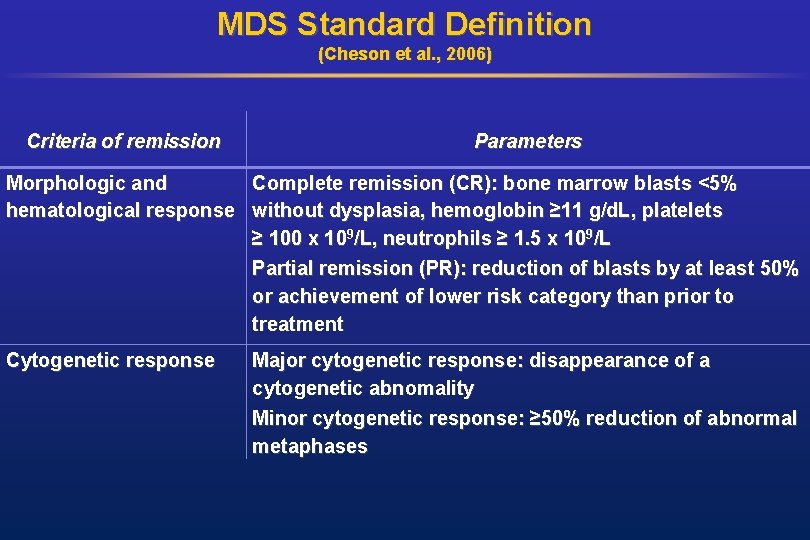
MDS Standard Definition (Cheson et al. , 2006) Criteria of remission Parameters Morphologic and Complete remission (CR): bone marrow blasts <5% hematological response without dysplasia, hemoglobin ≥ 11 g/d. L, platelets ≥ 100 x 109/L, neutrophils ≥ 1. 5 x 109/L Partial remission (PR): reduction of blasts by at least 50% or achievement of lower risk category than prior to treatment Cytogenetic response Major cytogenetic response: disappearance of a cytogenetic abnomality Minor cytogenetic response: ≥ 50% reduction of abnormal metaphases

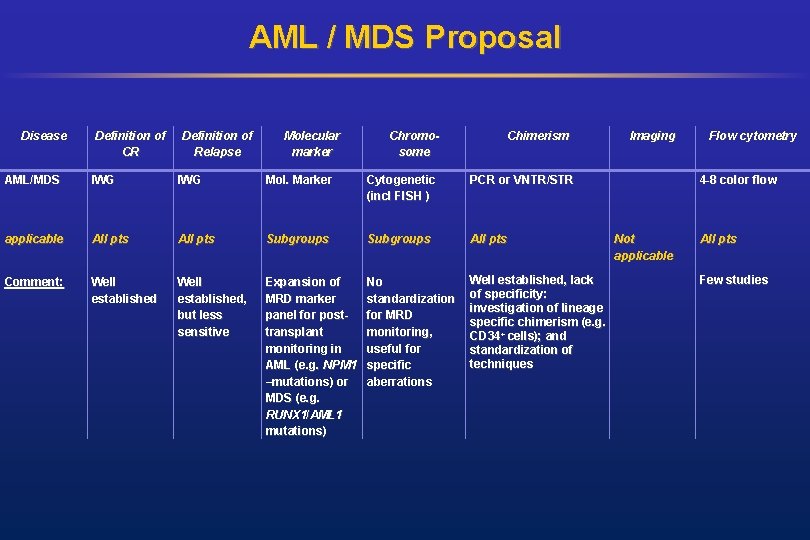
AML / MDS Proposal Disease Definition of CR Definition of Relapse Molecular marker Chromosome Chimerism AML/MDS IWG Mol. Marker Cytogenetic (incl FISH ) PCR or VNTR/STR applicable All pts Subgroups All pts Comment: Well established, but less sensitive Expansion of MRD marker panel for posttransplant monitoring in AML (e. g. NPM 1 –mutations) or MDS (e. g. RUNX 1/AML 1 mutations) No standardization for MRD monitoring, useful for specific aberrations Well established, lack of specificity: investigation of lineage specific chimerism (e. g. CD 34+ cells); and standardization of techniques Imaging Flow cytometry 4 -8 color flow Not applicable All pts Few studies

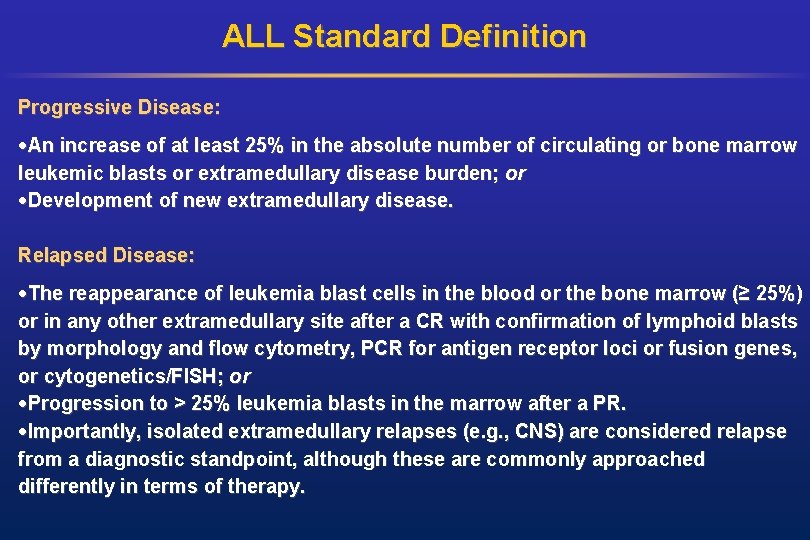
ALL Standard Definition Progressive Disease: An increase of at least 25% in the absolute number of circulating or bone marrow leukemic blasts or extramedullary disease burden; or Development of new extramedullary disease. Relapsed Disease: The reappearance of leukemia blast cells in the blood or the bone marrow (≥ 25%) or in any other extramedullary site after a CR with confirmation of lymphoid blasts by morphology and flow cytometry, PCR for antigen receptor loci or fusion genes, or cytogenetics/FISH; or Progression to > 25% leukemia blasts in the marrow after a PR. Importantly, isolated extramedullary relapses (e. g. , CNS) are considered relapse from a diagnostic standpoint, although these are commonly approached differently in terms of therapy.

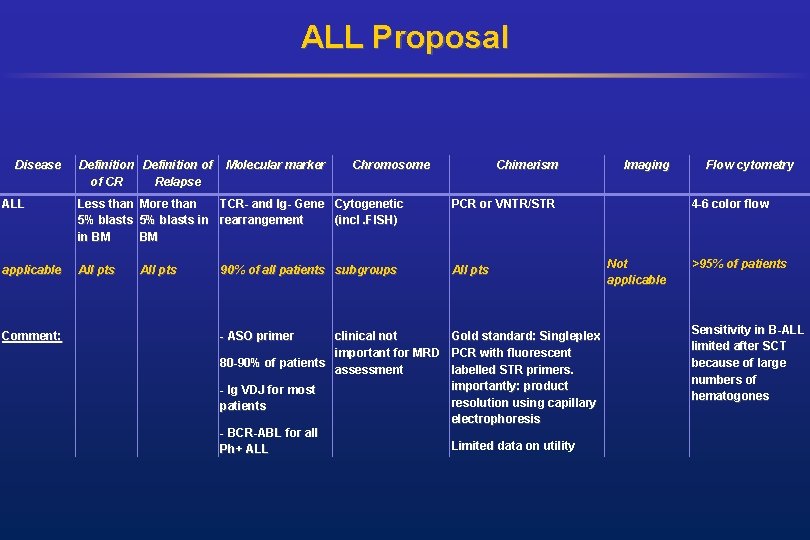
ALL Proposal Disease Definition of of CR Relapse Molecular marker Chromosome Chimerism ALL Less than More than TCR- and Ig- Gene Cytogenetic 5% blasts in rearrangement (incl. FISH) in BM BM PCR or VNTR/STR applicable All pts Comment: All pts 90% of all patients subgroups - ASO primer clinical not Gold standard: Singleplex important for MRD PCR with fluorescent 80 -90% of patients assessment labelled STR primers. importantly: product - Ig VDJ for most resolution using capillary patients electrophoresis - BCR-ABL for all Limited data on utility Ph+ ALL Imaging Flow cytometry 4 -6 color flow Not applicable >95% of patients Sensitivity in B-ALL limited after SCT because of large numbers of hematogones

CLL Standard Definition • Relapse: progression occurring 6 months or later after having achieved CR or PR • Progression: IW-CLL/NCI-WG criteria for CLL progression (at least one must apply) • Appearance of any new lesion such as enlarged lymph nodes (> 1. 5 cm), splenomegaly, hepatomegaly or other organ infiltrates; • increase of lymphadenopathy by 50% or more in greatest determined diameter of any previous site, or an increase of 50% or more in the sum of the product of diameters of multiple nodes; • increase in the liver or spleen size by 50% or more or the de novo appearance of hepatomegaly or splenomegaly; • increase in the number of blood lymphocytes by 50% or more with at least 5/n. L B cells; • transformation to a more aggressive histology (e. g. Richter's syndrome). • occurrence of cytopenia (neutropenia, anemia or thrombocytopenia) attributable to CLL. • Complete MRD response: clinical remission in the absence of one CLL cell per 10, 000 leukocytes in the peripheral blood or bone marrow • MRD relapse: Tumor cell recurrence or increases at the MRD level that does not exceed 5 B cells/n. L in the peripheral blood.

CLL Proposal Disease Definition of CR Definition of Relapse Molecular marker Chromosome Chimerism Imaging Flow cytometry CLL iw. CLL/NCI iw. CLL/NC ASO-primer IGH q. PCR Cytogenetic PCR/VNTR (incl FISH) CT MRD flow applicable All pts ~90% subgroup All pts > 95% Comment: iw. CLL definition of MRD negativity: predictive for No role in sustained remission relapse if < 10 -4 1 year post monitoring SCT. Only to be used if predictive for CR by clinical sustained remission methods or in if < 10 -4 1 year post clinical trials SCT. MRD < 10 -4 by q. PCR or MRD Flow More sensitive than MRD flow below Complete donor chimerism usually prerequisite for MRD negativity, but not suitable as MRD marker 10 -4 Equally sensitive and specific as q. PCR up to 10 -4

Lymphoma Standard Definition (Cheson et al. , 2007) Response CR Definition Nodal Masses Disappearance of (a) FDG-avid or PET positive prior to all evidence of therapy; mass of any size disease permitted if PET negative (b) Variably FDG-avid or PET negative; regression to normal size on CT Relapsed disease or PD Any new lesion or increase by ≥ 50 % of previously involved sites from nadir Spleen, Liver Not palpable, nodules disappeared Bone Marrow Infiltrate cleared on repeat biopsy; if indeterminate by morphology, immunohistochemistry should be negative Appearance of a new lesion(s) > 1. 5 cm > 50 % increase New or recurrent in any axis, ≥ 50 % increase in SPD of from nadir in the involvement more than one node, or ≥ 50 % SPD of any previous increase in longest diameter of a lesions previously identified node > 1 cm in short axis Lesions PET positive if FDG-avid lymphoma or PET positive prior to therapy

Lymphoma Proposal Disease Definition of CR Definition of Relapse Molecular marker Chromosome Chimerism Imaging Lymphoma Cheson criteria ASO-primer (Ig. H ) for Cytogenetic B-cell NHL (incl FISH) PCR or VNTR/STR CT/PET applicable All patient Comment: Well established Well Bcl-2 for FL t(14; 18) for FL Monitoring T-cell for all established for by PCR useful in Bcl-1 for about 30% of t(11, 14) for MCL lymphomas all lymphomas NHL. Role not MCL established in HD T cell receptor for TNHL subgroups Flow cytometry 4 -6 color flow All patient Subgroups Well established Could be helpful for FL and MCL in all lymphomas

Multiple Myeloma Standard Definition Relapse: EBMT criteria (Bladè et al) requires at least one of the following: • Reappearance of serum or urinary paraprotein on immunofixation or routine electrophoresis, confirmed by at least on further investigation and excluding oligoclonal immune reconstitution. • ≥ 5 % plasma cells in a bone marrow aspirate or on trephine bone biopsy. • Development of new lytic bone lesions or soft tissue plasmacytomas or definite increase in the size of residual bone lesions (development of a compression fracture does not exclude continued response and may not indicate progression). • Development of hypercalcaemia (corrected serum calcium > 11. 5 mg/dl or 2. 8 mmol/l) not attributable to any other cause. IWG Criteria (Durie et al): Relapse from CR requires at least one of the following: • Reappearance of serum or urinary M-protein by immunofixation or electrophoresis • ≥ 5 % plasma cells in a bone marrow. • Appearance of any other sign of progression (i. e new lytic bone lesions or soft tissue plasmacytomas or hypercalcemia).

Multiple Myeloma Proposal Disease Definition of CR Definition of Relapse Molecular marker Chromosome Chimerism Imaging Multiple Myeloma 1) EBMT 2) IWG ASO-primer (Ig. H) Cytogenetic (incl FISH) PCR or VNTR/STR MRI PET-CT 4 -8 color flow applicable All pts 40 -80% subgroups All pts Comment: Accepted but less sensitive Important, but May be useful* MNC-donor not included in chimerism not EBMT and IWG useful, lineage definition specific donor chimerism (CD 138+ plasma cells) predicts relapse Not established, but useful for extramedull ary More sensitive than EBMT/IWG in predicting relapse disease Flow cytometry Other methods Free light chain assay subgroups Proposed by IWG: no valid data

Sub-Committee on Disease-Specific Methods And Strategies For Monitoring Relapse Following Allogeneic Stem Cell Transplantation Panel Discussion

Relapse and Response Definitions After SCT Standard diagnostic criteria used to define response and relapse Ø Well validated in upfront clinical trials Ø Utility after allogeneic SCT is limited for most hematologic malignancies Sensitive disease-specific detection methods Ø Methodologic standardization and validation Ø Highly sensitive monitoring possible Ø Prognostic value in predicting continuous remission vs. relapse Ø Facilitate early intervention Ø Utility “pre-emptive” initiation of therapy prior to overt relapse Proposed incorporation of sensitive detection methods to augment standard response/relapse definitions for use in allogeneic SCT trials Ø Response endpoints Ø Relapse prediction Ø Relapse prevention Ø Relapse treatment

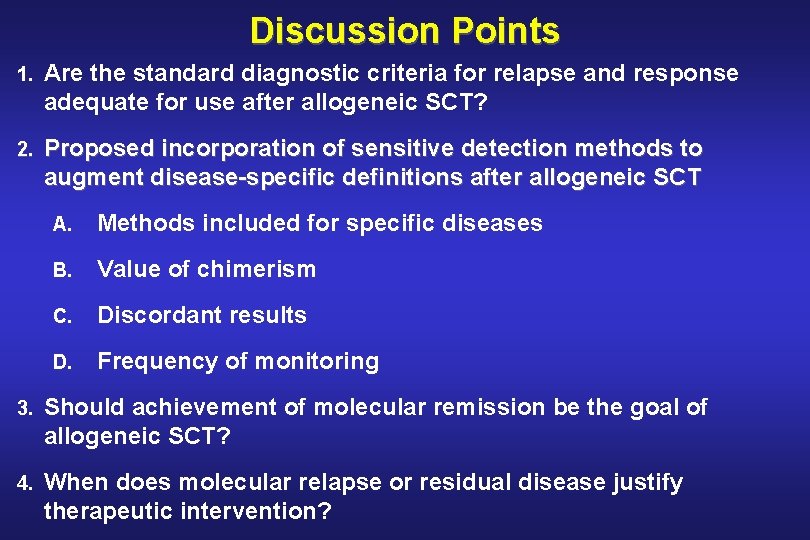
Discussion Points 1. Are the standard diagnostic criteria for relapse and response adequate for use after allogeneic SCT? 2. Proposed incorporation of sensitive detection methods to augment disease-specific definitions after allogeneic SCT A. Methods included for specific diseases B. Value of chimerism C. Discordant results D. Frequency of monitoring 3. Should achievement of molecular remission be the goal of allogeneic SCT? 4. When does molecular relapse or residual disease justify therapeutic intervention?

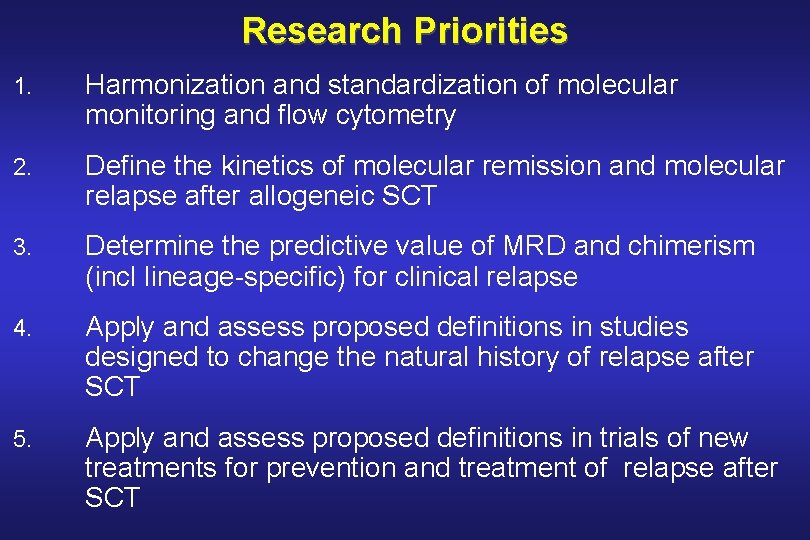
Research Priorities 1. Harmonization and standardization of molecular monitoring and flow cytometry 2. Define the kinetics of molecular remission and molecular relapse after allogeneic SCT 3. Determine the predictive value of MRD and chimerism (incl lineage-specific) for clinical relapse 4. Apply and assess proposed definitions in studies designed to change the natural history of relapse after SCT 5. Apply and assess proposed definitions in trials of new treatments for prevention and treatment of relapse after SCT
 Cycle of relapse and recovery
Cycle of relapse and recovery Cenaps relapse prevention model
Cenaps relapse prevention model Captasa
Captasa Nnn relapse
Nnn relapse Efficcity
Efficcity Research methods in monitoring and evaluation
Research methods in monitoring and evaluation Translation methods and strategies
Translation methods and strategies Unitless measure of productivity
Unitless measure of productivity Monitoring comprehension strategy
Monitoring comprehension strategy Monitoring customer service
Monitoring customer service Wax pattern in dentistry
Wax pattern in dentistry Fspos
Fspos Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidböcker
Tidböcker Sura för anatom
Sura för anatom Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Debatt artikel mall
Debatt artikel mall Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Lufttryck formel
Lufttryck formel Publik sektor
Publik sektor Bo bergman jag fryser om dina händer
Bo bergman jag fryser om dina händer Presentera för publik crossboss
Presentera för publik crossboss Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Vem räknas som jude
Vem räknas som jude Treserva lathund
Treserva lathund Epiteltyper
Epiteltyper Claes martinsson
Claes martinsson Cks
Cks Byggprocessen steg för steg
Byggprocessen steg för steg Bra mat för unga idrottare
Bra mat för unga idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Referatmarkering
Referatmarkering Redogör för vad psykologi är
Redogör för vad psykologi är Matematisk modellering eksempel
Matematisk modellering eksempel Atmosfr
Atmosfr Borra hål för knoppar
Borra hål för knoppar Orubbliga rättigheter
Orubbliga rättigheter Formel standardavvikelse
Formel standardavvikelse Tack för att ni har lyssnat
Tack för att ni har lyssnat Steg för steg rita
Steg för steg rita Vad är verksamhetsanalys
Vad är verksamhetsanalys Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Toppslätskivling effekt
Toppslätskivling effekt Mästar lärling modellen
Mästar lärling modellen Egg för emanuel
Egg för emanuel Elektronik för barn
Elektronik för barn Mantel som bars av kvinnor i antikens rom
Mantel som bars av kvinnor i antikens rom Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Kung som dog 1611
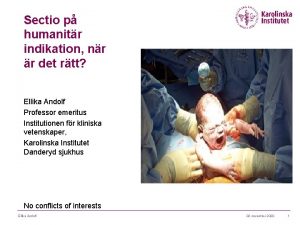
Kung som dog 1611 Indikation för kejsarsnitt på moderns önskan
Indikation för kejsarsnitt på moderns önskan Sju för caesar
Sju för caesar Tack för att ni lyssnade
Tack för att ni lyssnade Enheter för massa
Enheter för massa Bunden form
Bunden form Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Rbk fuktmätning
Rbk fuktmätning Etik och ledarskap etisk kod för chefer
Etik och ledarskap etisk kod för chefer Exspektans eller expektans
Exspektans eller expektans Myndigheten för delaktighet
Myndigheten för delaktighet Frgar
Frgar Sju principer för tillitsbaserad styrning
Sju principer för tillitsbaserad styrning Läkarutlåtande för livränta
Läkarutlåtande för livränta Stig karttecken
Stig karttecken Gumman cirkel sång
Gumman cirkel sång Shivaismen
Shivaismen