Discovery of Radioactivity Wilhelm Roentgen discovered that invisible
Discovery of Radioactivity ▪ Wilhelm Roentgen- discovered that invisible rays were emitted when electrons bombarded the surface of certain materials; name the rays→ X rays ▪ Henri Becqueral- discovered that phosphorescent uranium salt produced spontaneous emissions that darken photographic plates ▪ Maria Curie- took Becqueral’s mineral sample & isolated the components emitting the rays; concluded that the darkening of the photographic plates were due to rays emitted from the uranium atoms; name this process radioactivity; the rays & particles emitted by a radioactive source → radiation

▪ Radioactivity- some substances spontaneously emitted radiation. ▪ Radiation- rays and particles emitted by the radioactive material By emitting radiation, atoms on an element can change into atoms of another element ▪ Radioactive atoms emit radiation because their nuclei are unstable ▪
▪ Isotopes – same element that have different numbers of neutrons ▪ Radioisotopes- isotopes of atoms with unstable nuclei ▪ Release radiation to attain more stable atomic configuration → radioactive decay
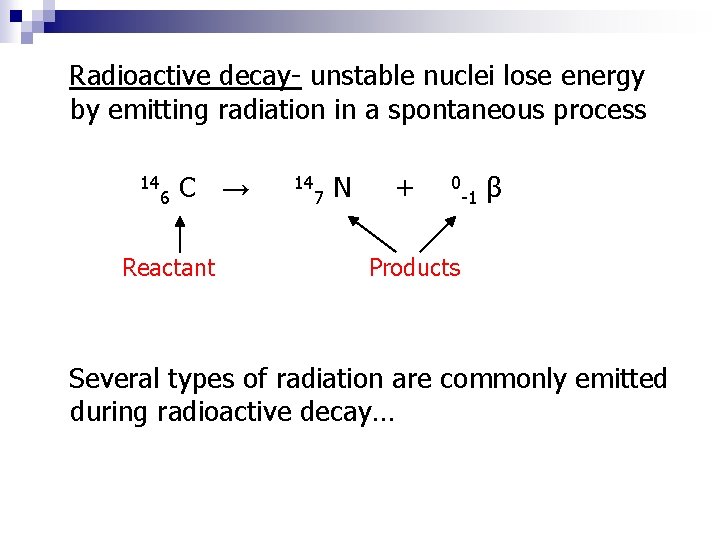
Radioactive decay- unstable nuclei lose energy by emitting radiation in a spontaneous process 14 6 C → Reactant 14 7 N + 0 -1 β Products Several types of radiation are commonly emitted during radioactive decay…
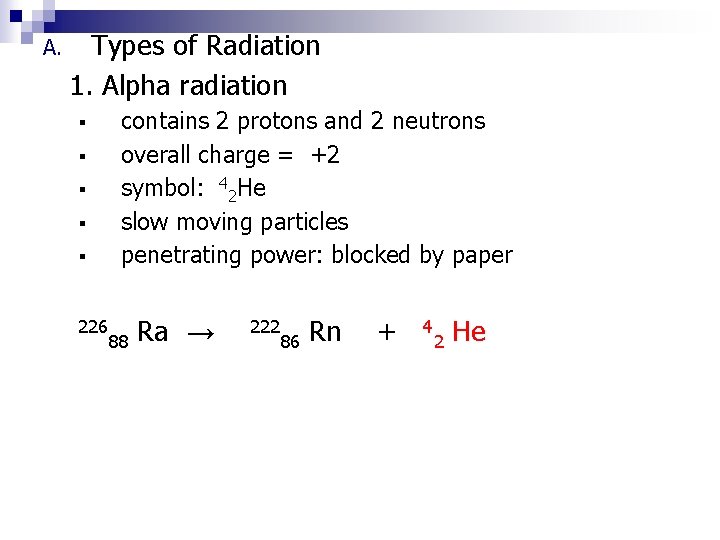
A. Types of Radiation 1. Alpha radiation ▪ ▪ ▪ 226 contains 2 protons and 2 neutrons overall charge = +2 symbol: 42 He slow moving particles penetrating power: blocked by paper 88 Ra → 222 86 Rn + 4 2 He
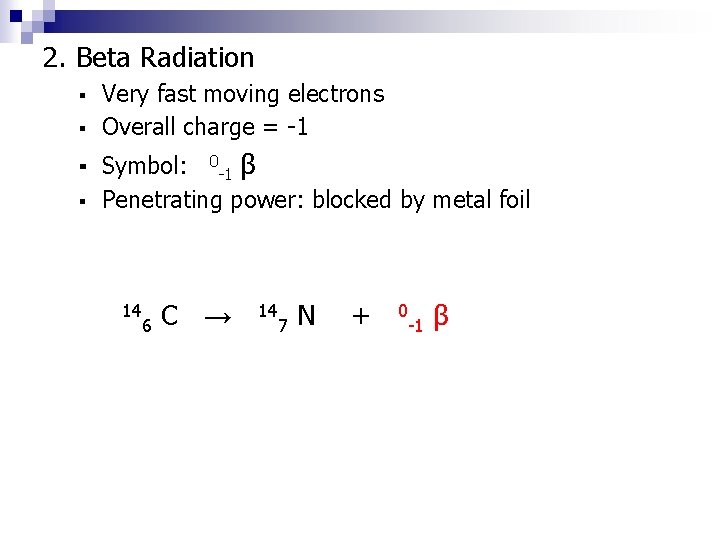
2. Beta Radiation Very fast moving electrons ▪ Overall charge = -1 ▪ ▪ Symbol: ▪ 0 -1 β Penetrating power: blocked by metal foil 14 6 C → 14 7 N + 0 -1 β
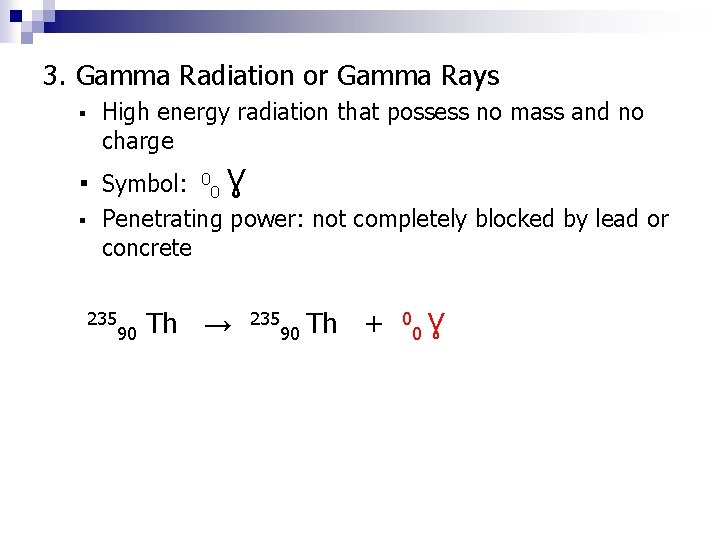
3. Gamma Radiation or Gamma Rays ▪ High energy radiation that possess no mass and no charge ▪ Symbol: ▪ 0 0 Ɣ Penetrating power: not completely blocked by lead or concrete 235 90 Th → 235 90 Th + 0 0 Ɣ

Penetration:

Alpha, Beta and Gamma passing through an electric field: + (Positive end attracts to the negative end)
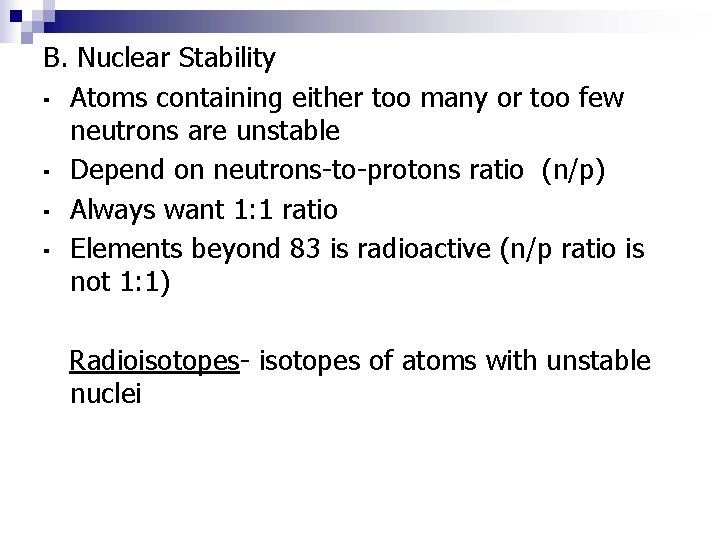
B. Nuclear Stability ▪ Atoms containing either too many or too few neutrons are unstable ▪ Depend on neutrons-to-protons ratio (n/p) ▪ Always want 1: 1 ratio ▪ Elements beyond 83 is radioactive (n/p ratio is not 1: 1) Radioisotopes- isotopes of atoms with unstable nuclei
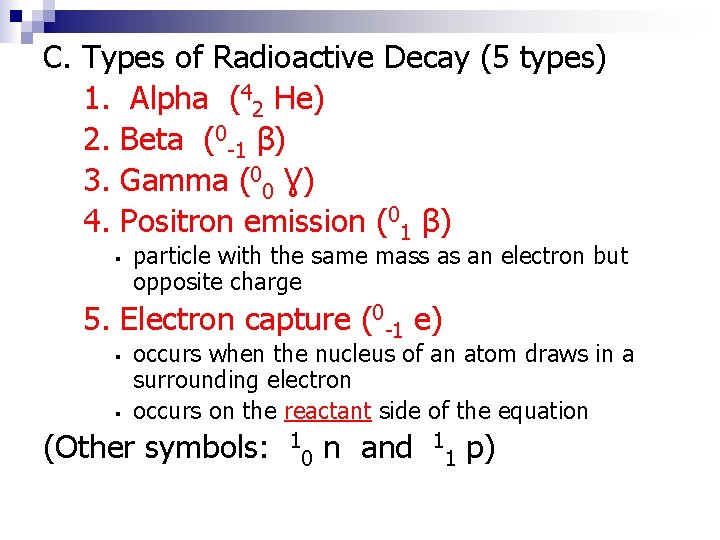
C. Types of Radioactive Decay (5 types) 1. Alpha (42 He) 2. Beta (0 -1 β) 3. Gamma (00 Ɣ) 4. Positron emission (01 β) ▪ particle with the same mass as an electron but opposite charge 5. Electron capture (0 -1 e) ▪ ▪ occurs when the nucleus of an atom draws in a surrounding electron occurs on the reactant side of the equation (Other symbols: 1 0 n and 1 1 p)
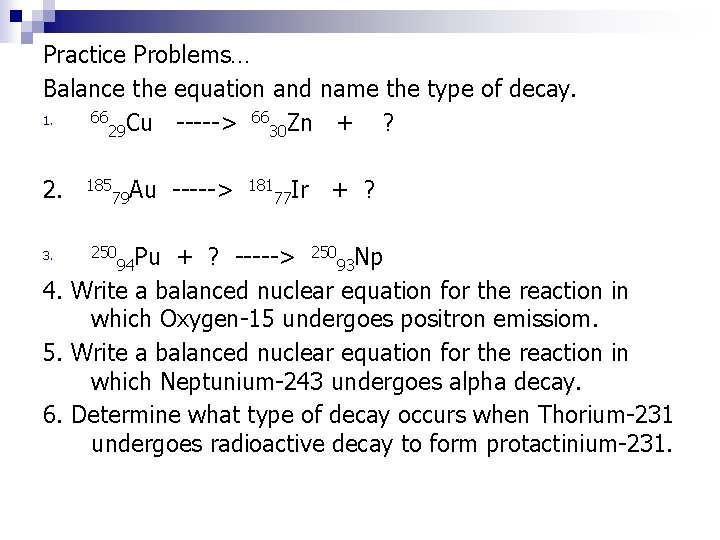
Practice Problems… Balance the equation and name the type of decay. 66 Cu -----> 66 Zn + 1. ? 29 30 2. 185 79 Au 181 77 Ir + ? -----> 25093 Np 4. Write a balanced nuclear equation for the reaction in which Oxygen-15 undergoes positron emissiom. 5. Write a balanced nuclear equation for the reaction in which Neptunium-243 undergoes alpha decay. 6. Determine what type of decay occurs when Thorium-231 undergoes radioactive decay to form protactinium-231. 3. 250 94 Pu ----->
- Slides: 12