Discovering the Atom Greek Model To understand the









- Slides: 23

Discovering the Atom

Greek Model “To understand the very large, we must understand the very small. ” Democritus • Greek philosopher (460 B. C. - 370 B. C. ) • Idea of ‘democracy’ • Idea of ‘atomos’ – Atomos = ‘indivisible’ – ‘Atom’ is derived • No experiments to support idea Democritus’ model of atom No protons, electrons, or neutrons Solid and INDESTRUCTABLE

Dalton’s Atomic Theory 1803 1. All matter is made of tiny indivisible particles called atoms. 2. Atoms of the same element are identical, those of different atoms are different. 3. Atoms of different elements combine in whole number ratios to form compounds 4. Chemical reactions involve the rearrangement of atoms. No new atoms are created or destroyed.

Radioactivity • One of the pieces of evidence for the fact that atoms are made of smaller particles came from the work of Marie Curie (1876 - 1934). • She discovered radioactivity, the spontaneous disintegration of some elements into smaller pieces.

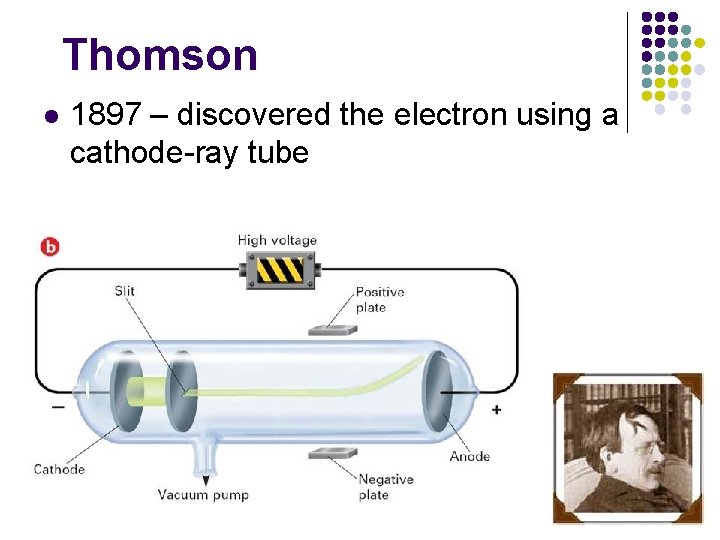
Thomson l 1897 – discovered the electron using a cathode-ray tube

Cathode-ray Tube Animation l http: //www. youtube. com/watch? v=1 iw 0 Plrk 51 Y

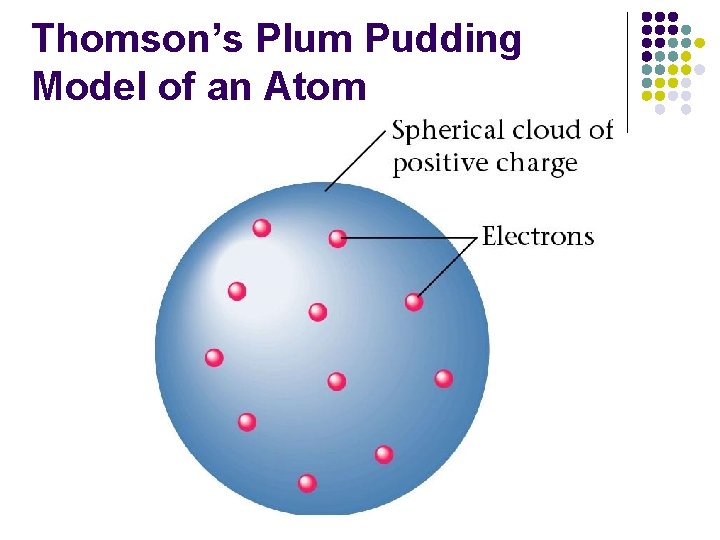
Thomson’s Plum Pudding Model of an Atom

Millikan l l l http: //www. youtube. com/watch? v=XMf. YHag 7 Liw Determined the charge of an electron Oil Drop Experiment

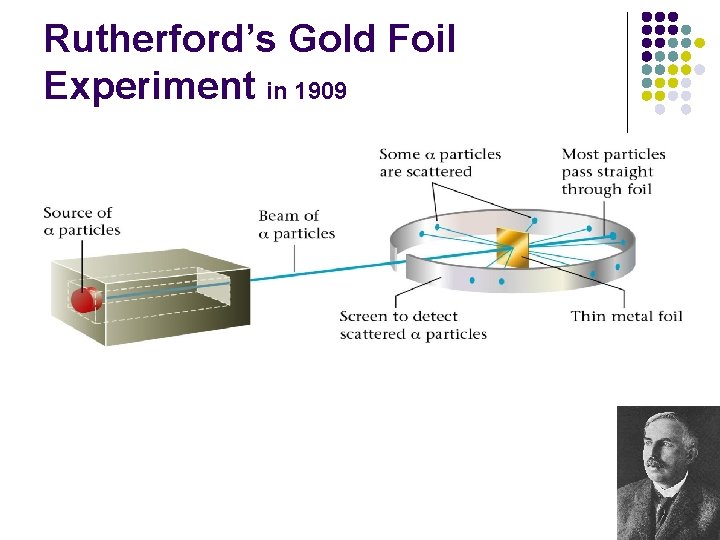
Rutherford’s Gold Foil Experiment in 1909

Rutherford’s Gold Foil Exp l http: //www. youtube. com/watch? v=5 p. Zj 0 u_X Mbc

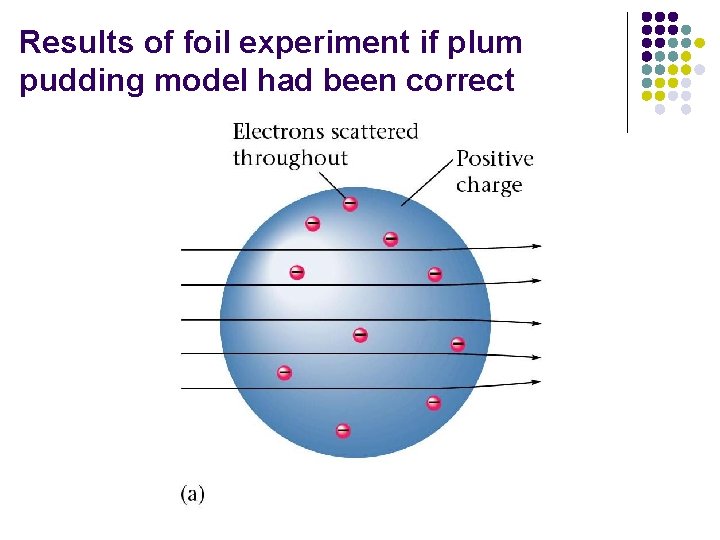
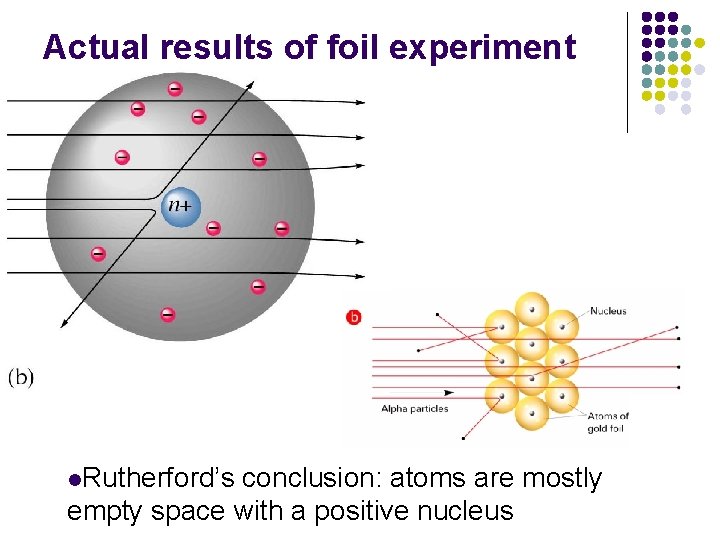
Results of foil experiment if plum pudding model had been correct

Actual results of foil experiment l. Rutherford’s conclusion: atoms are mostly empty space with a positive nucleus

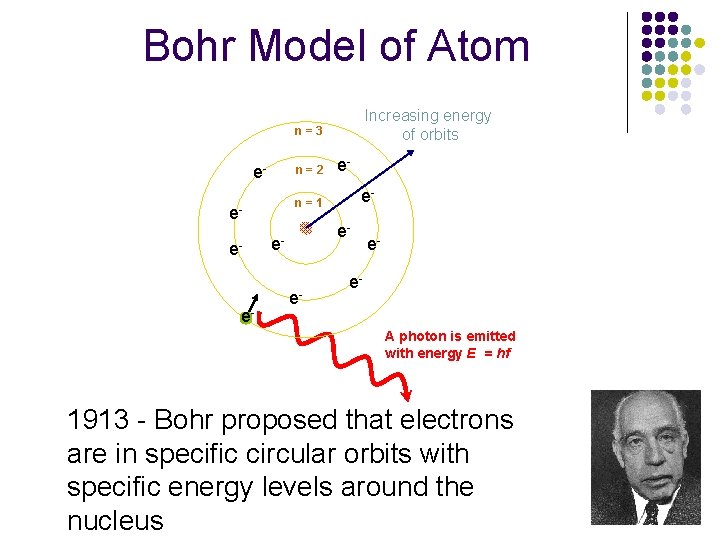
Bohr Model of Atom Increasing energy of orbits n=3 e- n=2 e- e- n=1 ee- e- e- e. A photon is emitted with energy E = hf 1913 - Bohr proposed that electrons are in specific circular orbits with specific energy levels around the nucleus

Electrons as Waves • Louis de Broglie (1924) – Applied wave-particle theory to electrons – electrons exhibit wave properties Fundamental mode 200 150 100 50 0 - 50 -100 -150 -200 0 50 100 150 Second Harmonic or First Overtone 200 150 100 50 0 - 50 -100 -150 -200 0 50 100 150 200 Standing Wave 200 150 100 50 0 - 50 -100 -150 -200 0 50 100 150 200

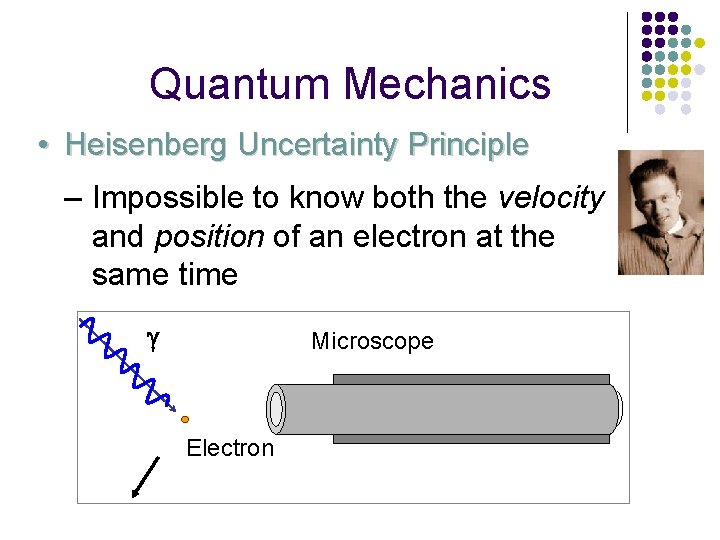
Quantum Mechanics • Heisenberg Uncertainty Principle – Impossible to know both the velocity and position of an electron at the same time g Microscope Electron

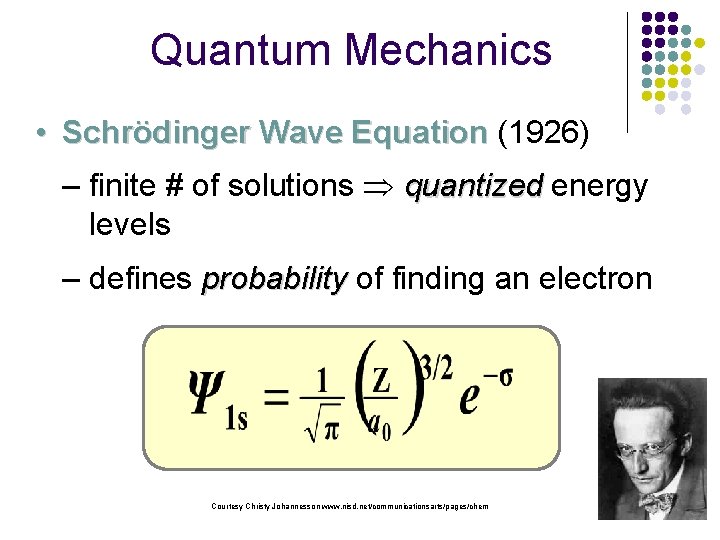
Quantum Mechanics • Schrödinger Wave Equation (1926) – finite # of solutions quantized energy levels – defines probability of finding an electron Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

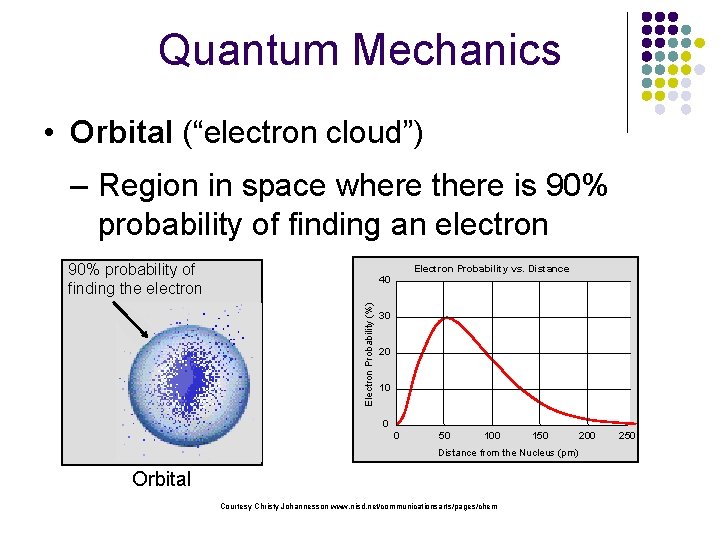
Quantum Mechanics • Orbital (“electron cloud”) – Region in space where there is 90% probability of finding an electron 90% probability of finding the electron Electron Probability vs. Distance Electron Probability (%) 40 30 20 10 0 0 50 100 150 Distance from the Nucleus (pm) Orbital Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 200 250

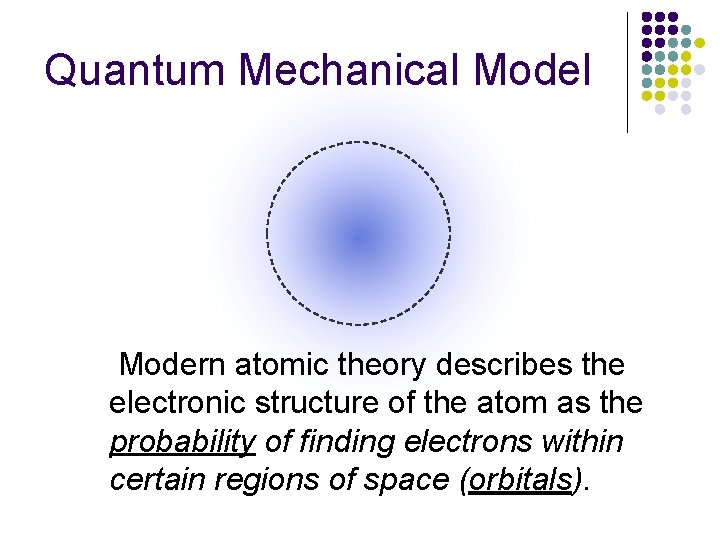
Quantum Mechanical Modern atomic theory describes the electronic structure of the atom as the probability of finding electrons within certain regions of space (orbitals).

Chadwick l http: //www. youtube. com/watch? v=ZK-yeuu_p 9 k l Confirmed existence of neutron l Start @ 3 min

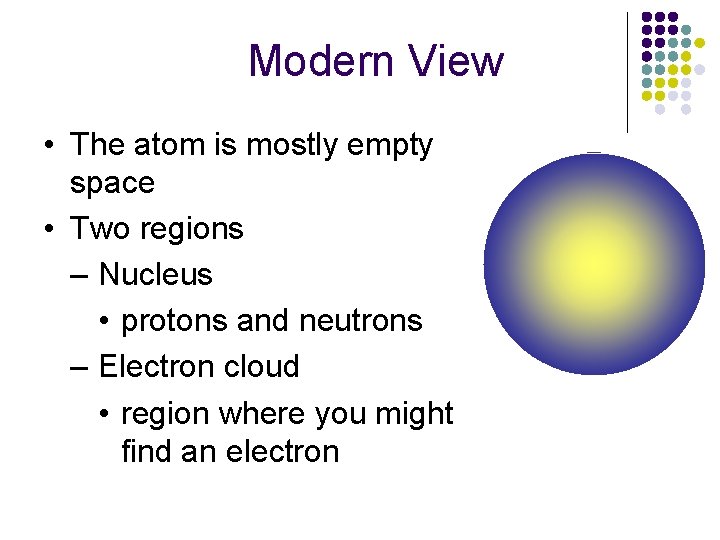
Modern View • The atom is mostly empty space • Two regions – Nucleus • protons and neutrons – Electron cloud • region where you might find an electron

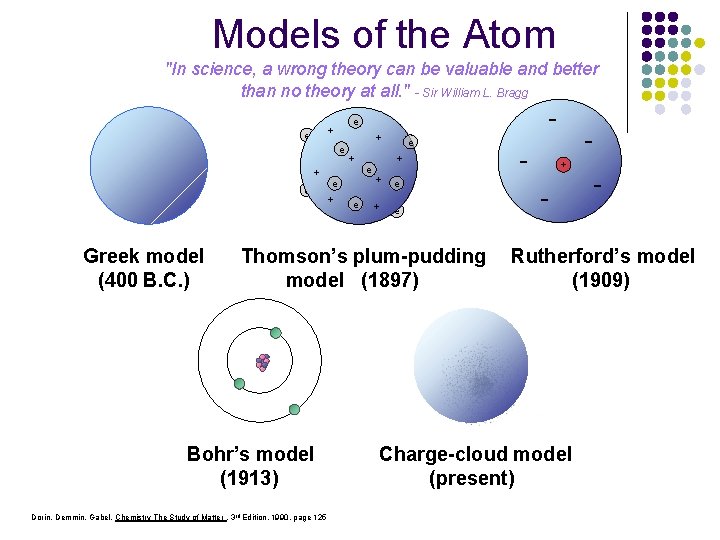
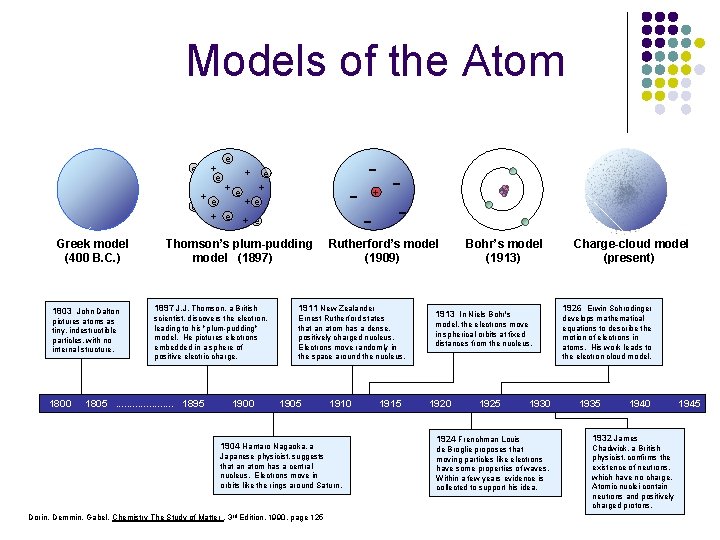
Models of the Atom "In science, a wrong theory can be valuable and better than no theory at all. " - Sir William L. Bragg + e + Dalton’smodel Greek (1803) (400 B. C. ) e + e + e - e + e + e Thomson’s plum-pudding model (1897) Bohr’s model (1913) Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 125 - + - - Rutherford’s model (1909) Charge-cloud model (present)

Models of the Atom e + e e - + e +e +e e + e Dalton’s model Greek model (1803) (400 B. C. ) 1803 John Dalton pictures atoms as tiny, indestructible particles, with no internal structure. 1800 - Thomson’s plum-pudding model (1897) - + Rutherford’s model (1909) 1897 J. J. Thomson, a British 1911 New Zealander scientist, discovers the electron, leading to his "plum-pudding" model. He pictures electrons embedded in a sphere of positive electric charge. Ernest Rutherford states that an atom has a dense, positively charged nucleus. Electrons move randomly in the space around the nucleus. 1805. . . . . 1895 1900 1905 1910 1904 Hantaro Nagaoka, a Japanese physicist, suggests that an atom has a central nucleus. Electrons move in orbits like the rings around Saturn. Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 125 1915 Bohr’s model (1913) 1926 Erwin Schrodinger 1913 In Niels Bohr's model, the electrons move in spherical orbits at fixed distances from the nucleus. 1920 1925 Charge-cloud model (present) 1930 1924 Frenchman Louis de Broglie proposes that moving particles like electrons have some properties of waves. Within a few years evidence is collected to support his idea. develops mathematical equations to describe the motion of electrons in atoms. His work leads to the electron cloud model. 1935 1940 1932 James Chadwick, a British physicist, confirms the existence of neutrons, which have no charge. Atomic nuclei contain neutrons and positively charged protons. 1945

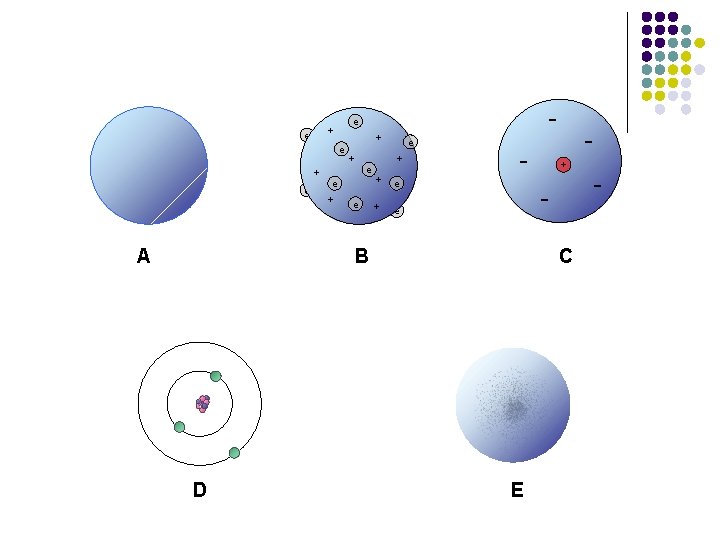
+ e + Dalton’s A model D e + e + e - + - B C E -