Disaccharides Disaccharides Formed from two monosaccharides Joined by

Disaccharides
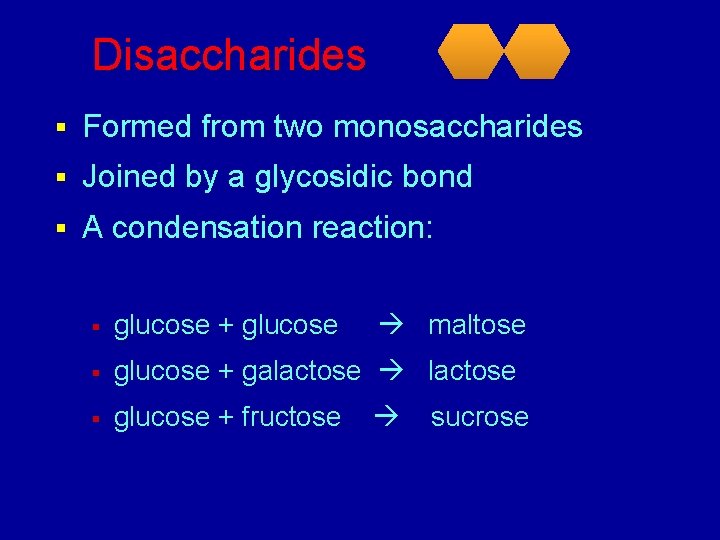
Disaccharides § Formed from two monosaccharides § Joined by a glycosidic bond § A condensation reaction: § glucose + glucose maltose § glucose + galactose § glucose + fructose sucrose
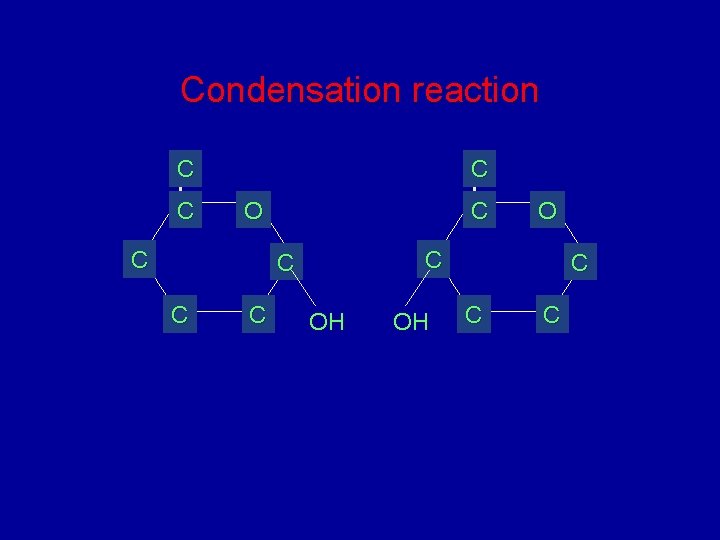
Condensation reaction C C C O OH OH C C C
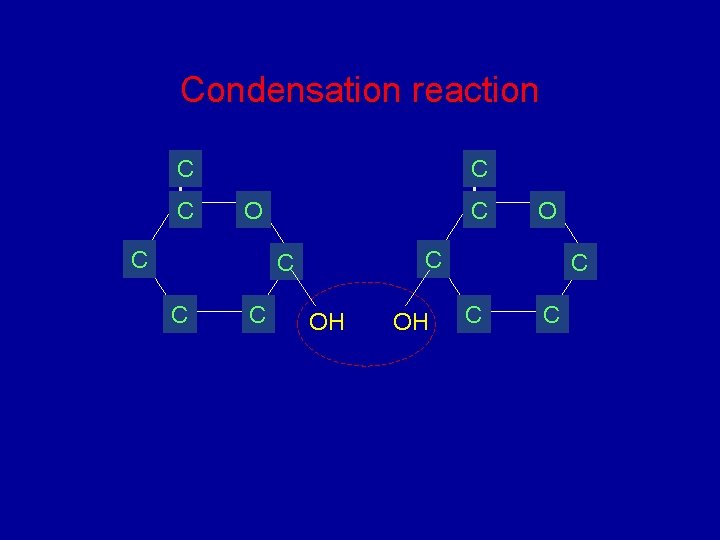
Condensation reaction C C C O OH OH C C C
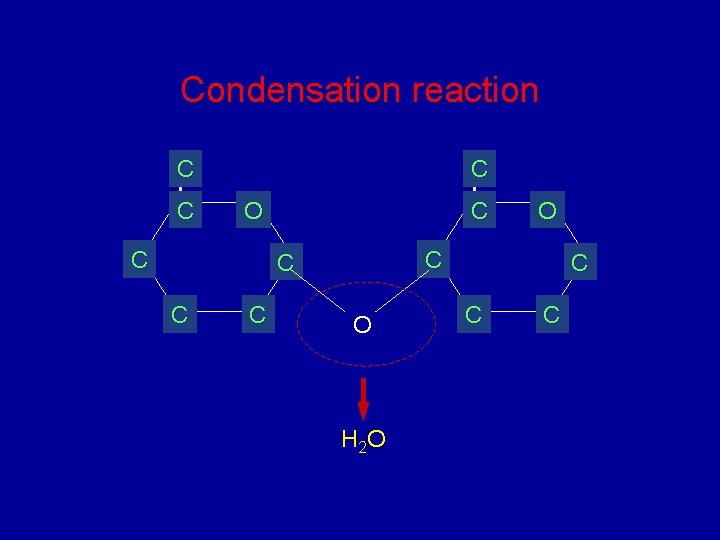
Condensation reaction C C C O O H 2 O C C C
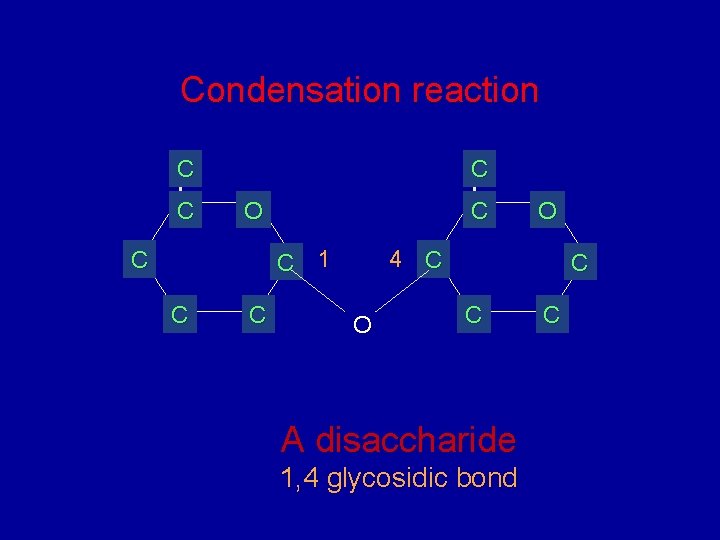
Condensation reaction C C C O C C 1 C C C O 4 C O C C A disaccharide 1, 4 glycosidic bond C
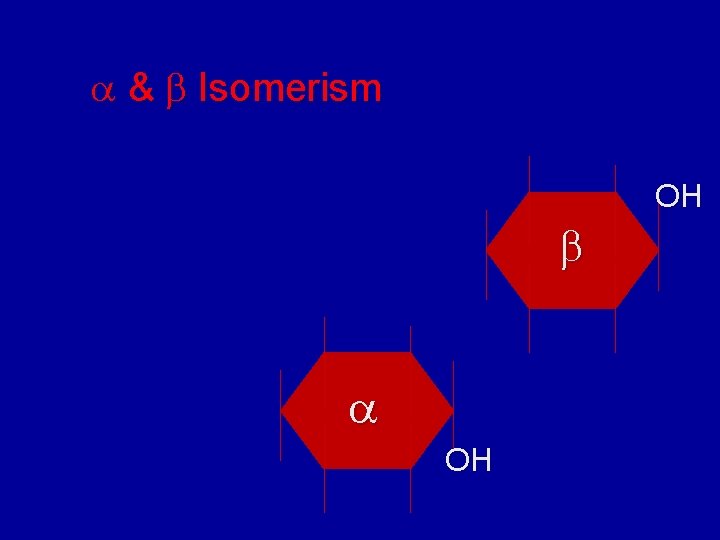
& Isomerism OH

and Anomers for D-Glucose The new –OH on C 1 is drawn down for the anomer, and up for the anomer. -D-Glucose
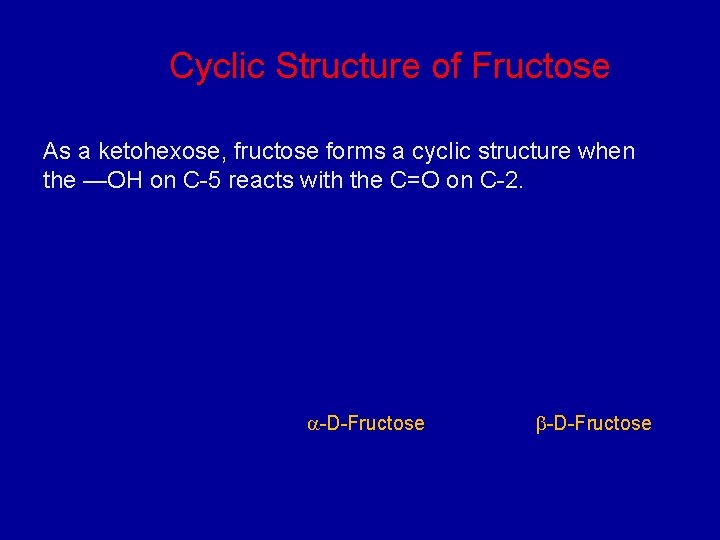
Cyclic Structure of Fructose As a ketohexose, fructose forms a cyclic structure when the —OH on C-5 reacts with the C=O on C-2. -D-Fructose
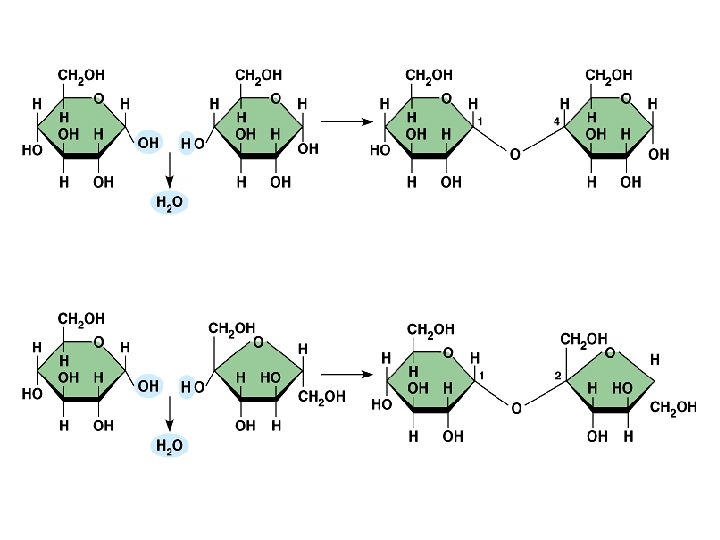
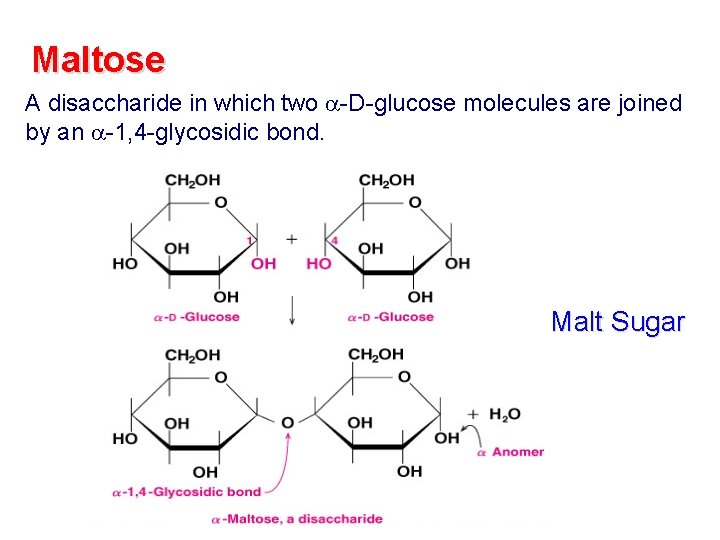
Maltose A disaccharide in which two -D-glucose molecules are joined by an -1, 4 -glycosidic bond. Malt Sugar
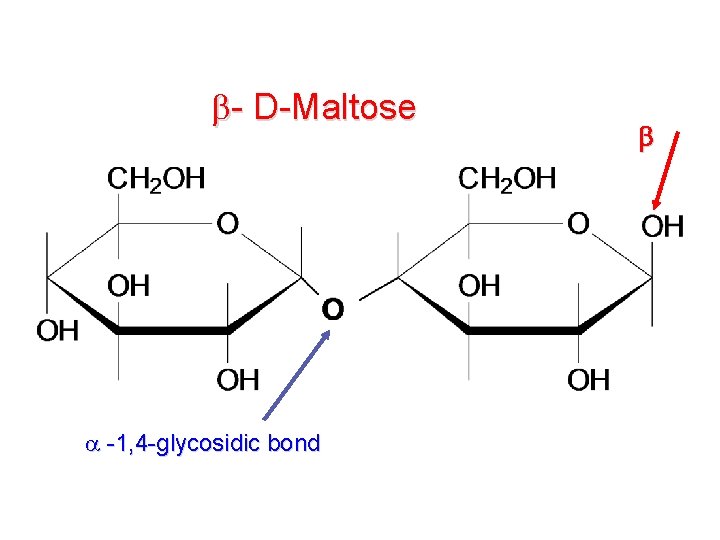
- D-Maltose -1, 4 -glycosidic bond
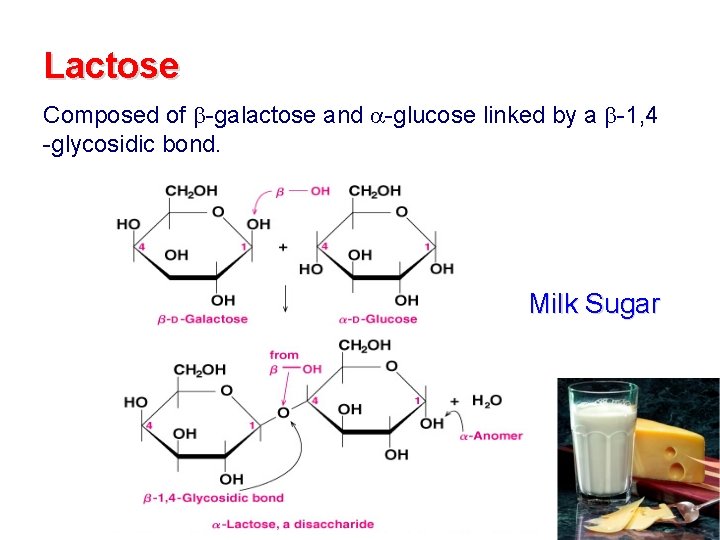
Lactose Composed of -galactose and -glucose linked by a -1, 4 -glycosidic bond. Milk Sugar
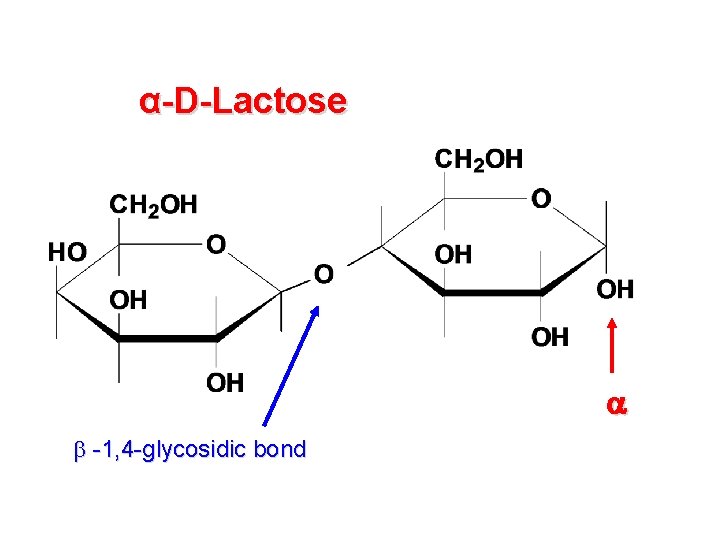
α-D-Lactose -1, 4 -glycosidic bond
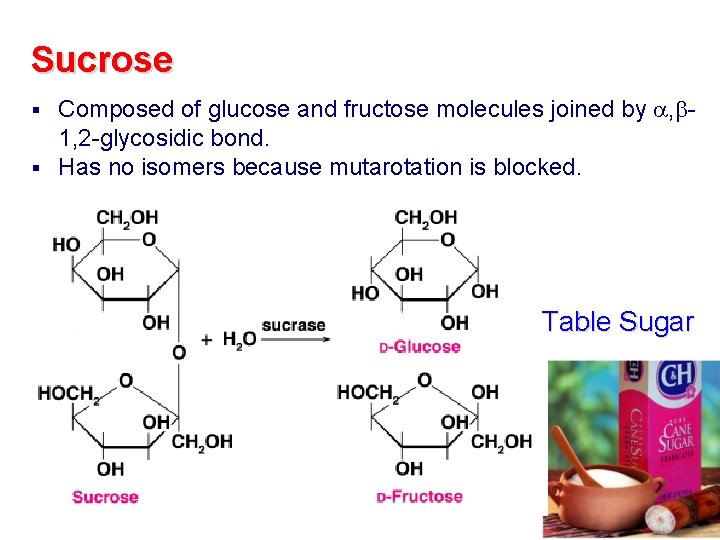
Sucrose Composed of glucose and fructose molecules joined by , 1, 2 -glycosidic bond. § Has no isomers because mutarotation is blocked. § Table Sugar
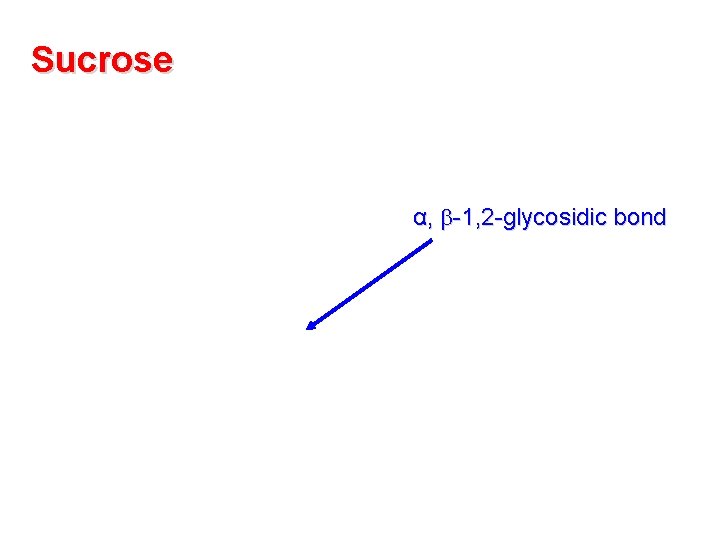
Sucrose α, -1, 2 -glycosidic bond
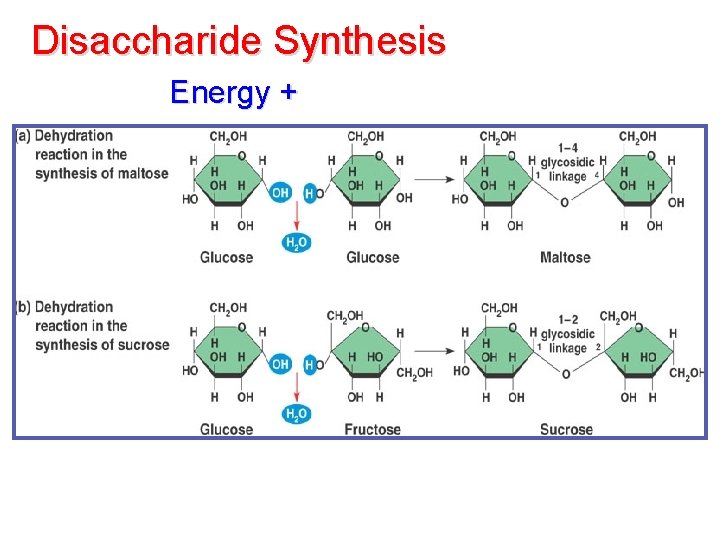
Disaccharide Synthesis Energy +
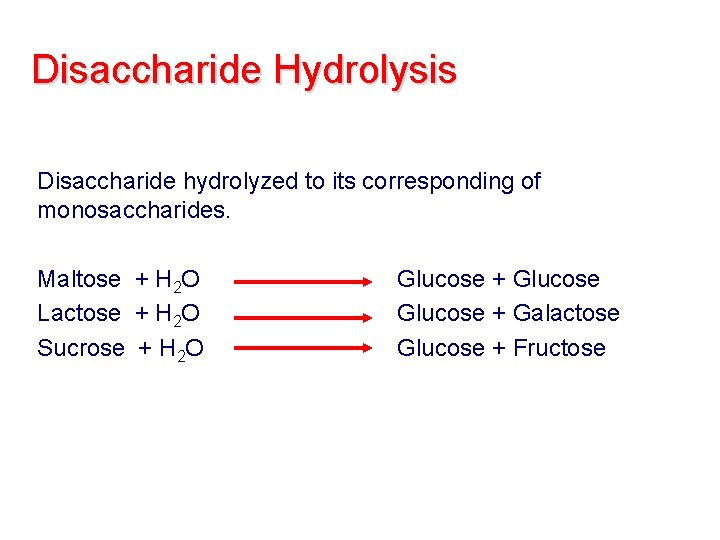
Disaccharide Hydrolysis Disaccharide hydrolyzed to its corresponding of monosaccharides. Maltose + H 2 O Lactose + H 2 O Sucrose + H 2 O Glucose + Galactose Glucose + Fructose
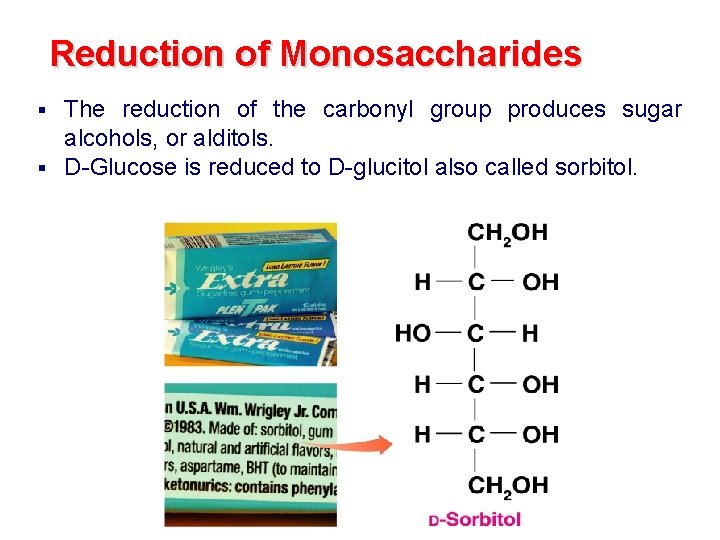
Reduction of Monosaccharides The reduction of the carbonyl group produces sugar alcohols, or alditols. § D-Glucose is reduced to D-glucitol also called sorbitol. §
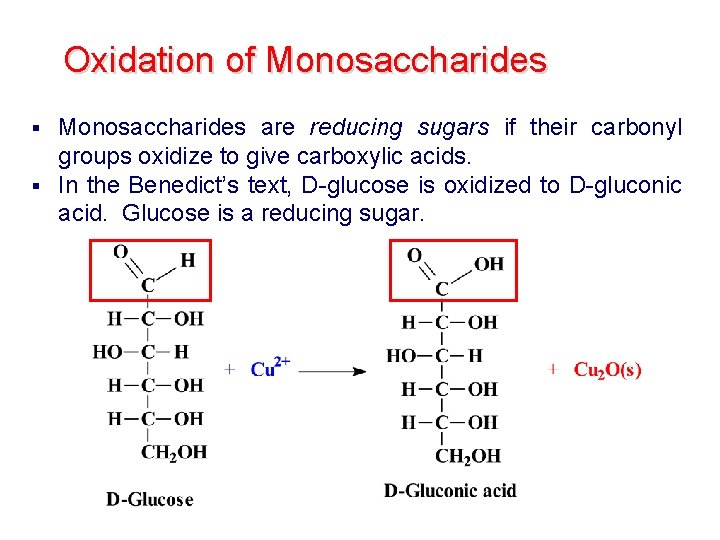
Oxidation of Monosaccharides are reducing sugars if their carbonyl groups oxidize to give carboxylic acids. § In the Benedict’s text, D-glucose is oxidized to D-gluconic acid. Glucose is a reducing sugar. §
- Slides: 20