Dipole Forces Main Concept Dipole forces result from


- Slides: 10

Dipole Forces Main Concept: Dipole forces result from the attraction among the positive ends and negative ends of polar molecules. Hydrogen bonding is a strong type of dipole-dipole force that exists when very electronegative atoms (N, O, and F) are involved.

Dipole Forces Dipoles Definition Causes Polar vs. Nonpolar Interactions Types of Dipoles Hydrogen Bonding Dipole-Dipole-Induced Dipole Definition Causes

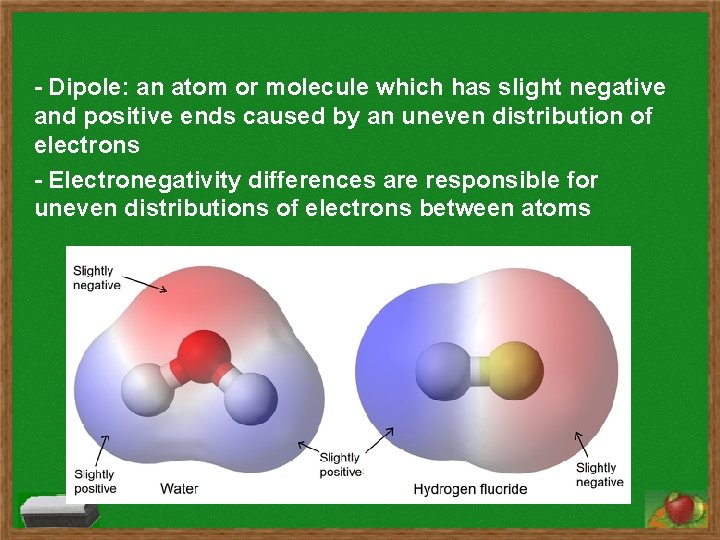
- Dipole: an atom or molecule which has slight negative and positive ends caused by an uneven distribution of electrons - Electronegativity differences are responsible for uneven distributions of electrons between atoms

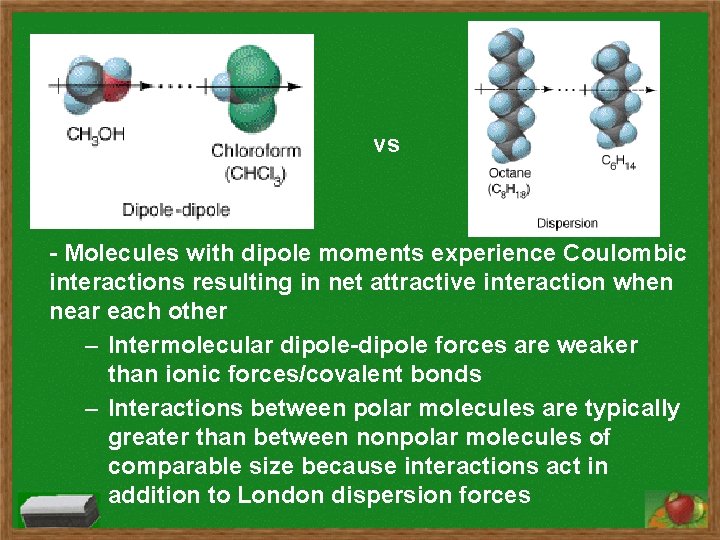
vs - Molecules with dipole moments experience Coulombic interactions resulting in net attractive interaction when near each other – Intermolecular dipole-dipole forces are weaker than ionic forces/covalent bonds – Interactions between polar molecules are typically greater than between nonpolar molecules of comparable size because interactions act in addition to London dispersion forces

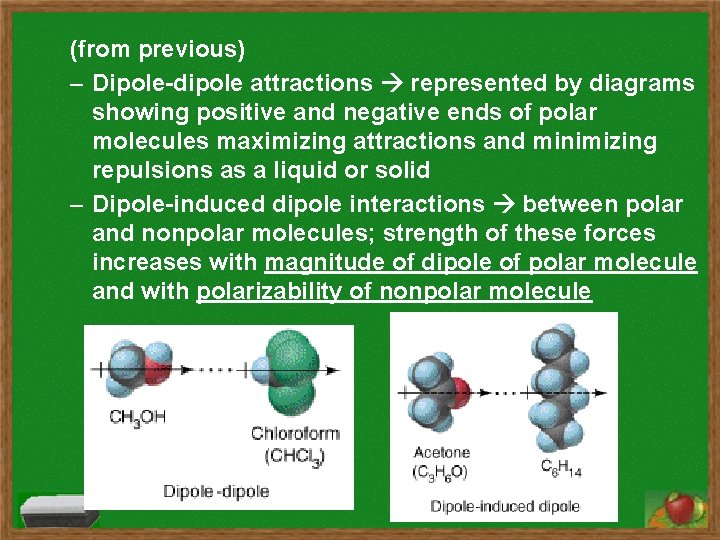
(from previous) – Dipole-dipole attractions represented by diagrams showing positive and negative ends of polar molecules maximizing attractions and minimizing repulsions as a liquid or solid – Dipole-induced dipole interactions between polar and nonpolar molecules; strength of these forces increases with magnitude of dipole of polar molecule and with polarizability of nonpolar molecule

Question: Order the following in terms of which would have a greater magnitude of dipole and explain why you think this. HF vs H 2 O vs NH 3

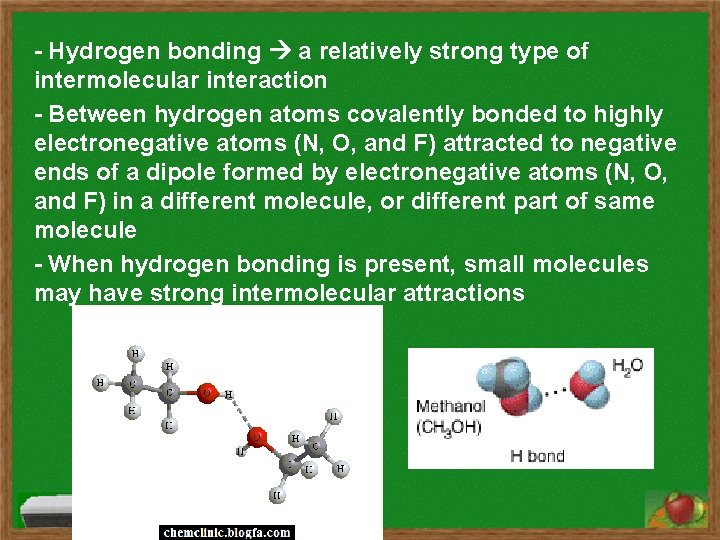
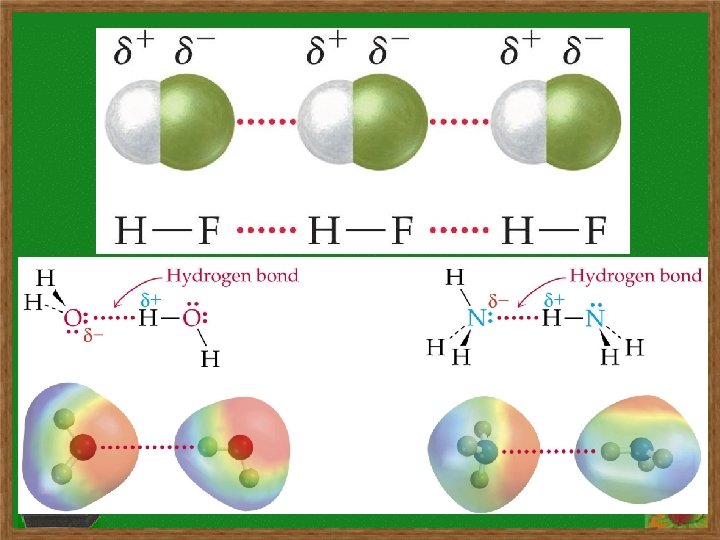
- Hydrogen bonding a relatively strong type of intermolecular interaction - Between hydrogen atoms covalently bonded to highly electronegative atoms (N, O, and F) attracted to negative ends of a dipole formed by electronegative atoms (N, O, and F) in a different molecule, or different part of same molecule - When hydrogen bonding is present, small molecules may have strong intermolecular attractions


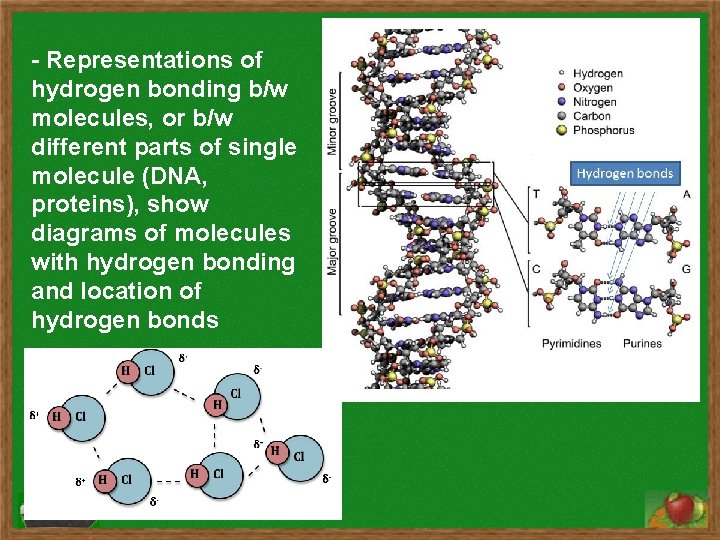
- Representations of hydrogen bonding b/w molecules, or b/w different parts of single molecule (DNA, proteins), show diagrams of molecules with hydrogen bonding and location of hydrogen bonds

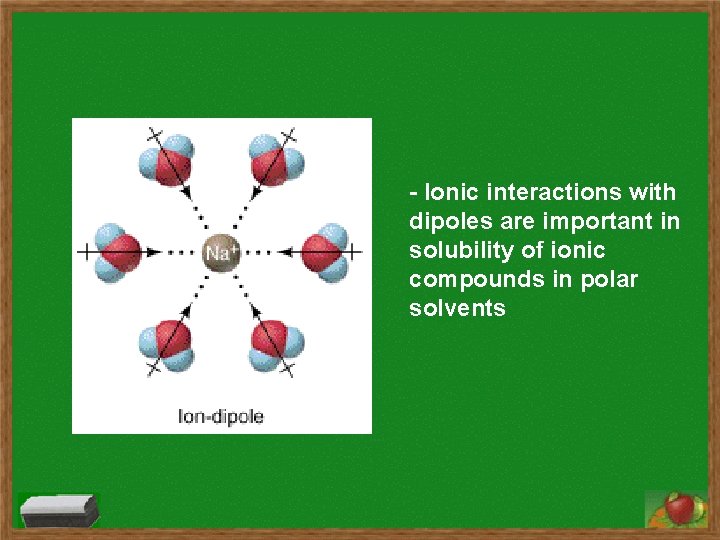
- Ionic interactions with dipoles are important in solubility of ionic compounds in polar solvents