Dimensions Unit Conversions August 10 11 2015 Dimensions








- Slides: 17

Dimensions & Unit Conversions August 10 / 11, 2015

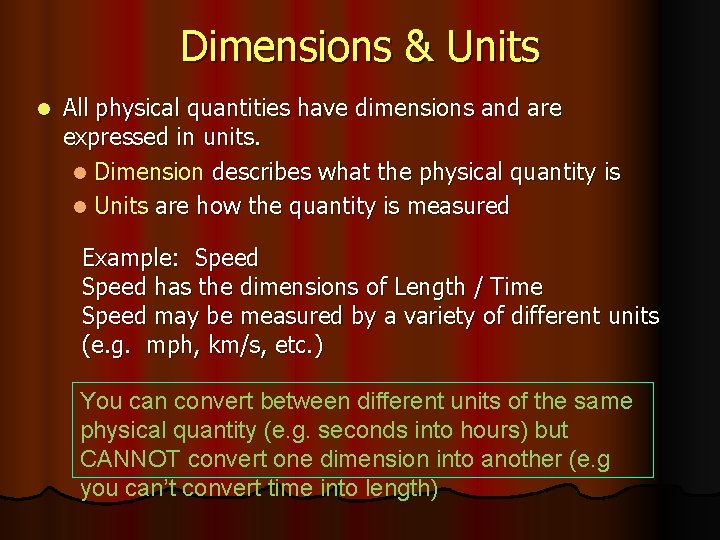
Dimensions & Units l All physical quantities have dimensions and are expressed in units. l Dimension describes what the physical quantity is l Units are how the quantity is measured Example: Speed has the dimensions of Length / Time Speed may be measured by a variety of different units (e. g. mph, km/s, etc. ) You can convert between different units of the same physical quantity (e. g. seconds into hours) but CANNOT convert one dimension into another (e. g you can’t convert time into length)

In the study of mechanics, we will work with physical quantities that can be described in terms of three dimensions: length (L), time (T) , and mass (M). The corresponding basic SI- units are: v. Length – 1 meter (1 m) is the distance traveled by the light in a vacuum during a time of 1/299, 792, 458 second. v. Mass – 1 kilogram (1 kg) is defined as a mass of a specific platinum-iridium alloy cylinder kept at the International Bureau of Weights and Measures at Sevres, France Time – 1 second (1 s) is defined as 9, 192, 631, 770 times the period of oscillation of radiation from the cesium atom.

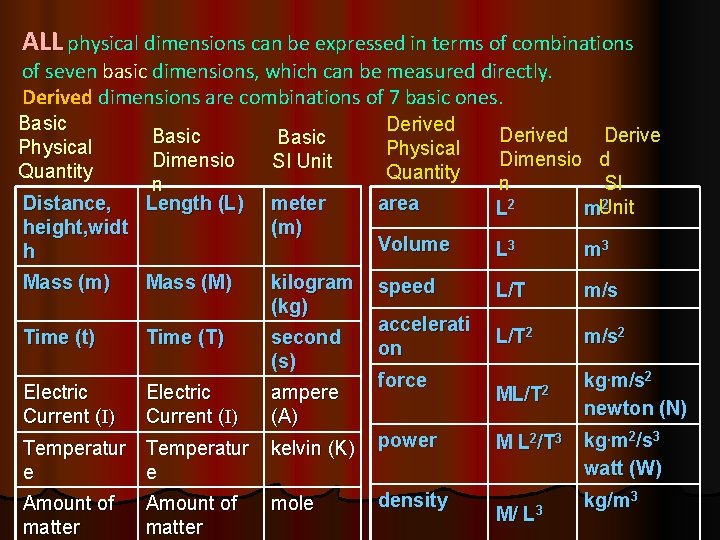
ALL physical dimensions can be expressed in terms of combinations of seven basic dimensions, which can be measured directly. Derived dimensions are combinations of 7 basic ones. Basic Physical Quantity Distance, height, widt h Mass (m) Time (t) Electric Current (I) Basic Dimensio n Length (L) Mass (M) Time (T) Electric Current (I) Derived Physical Quantity meter (m) area Derived Derive Dimensio d n SI 2 L 2 m. Unit Volume L 3 m 3 kilogram (kg) speed L/T m/s accelerati on L/T 2 m/s 2 ML/T 2 kg. m/s 2 newton (N) Basic SI Unit second (s) ampere (A) force Temperatur e e kelvin (K) power Amount of matter mole density Amount of matter M L 2/T 3 M/ L 3 kg. m 2/s 3 watt (W) kg/m 3

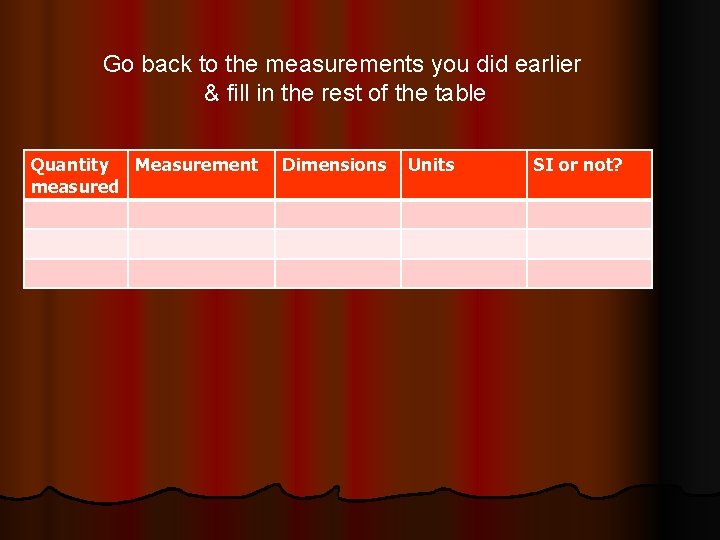
Go back to the measurements you did earlier & fill in the rest of the table Quantity Measurement measured Dimensions Units SI or not?

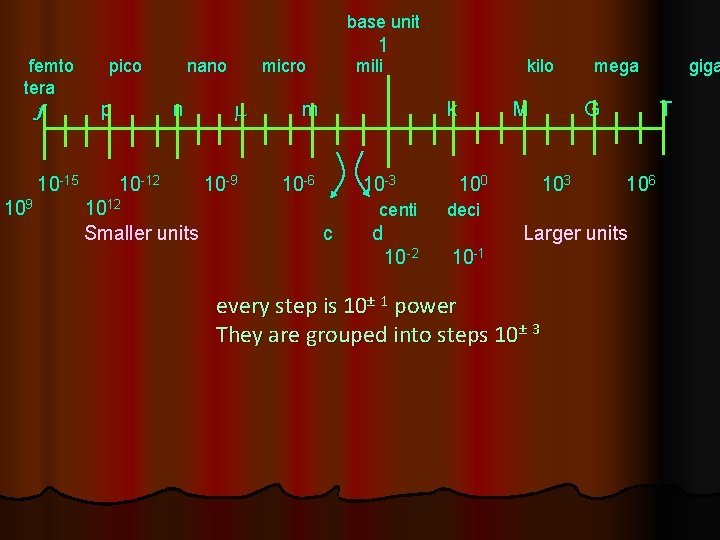
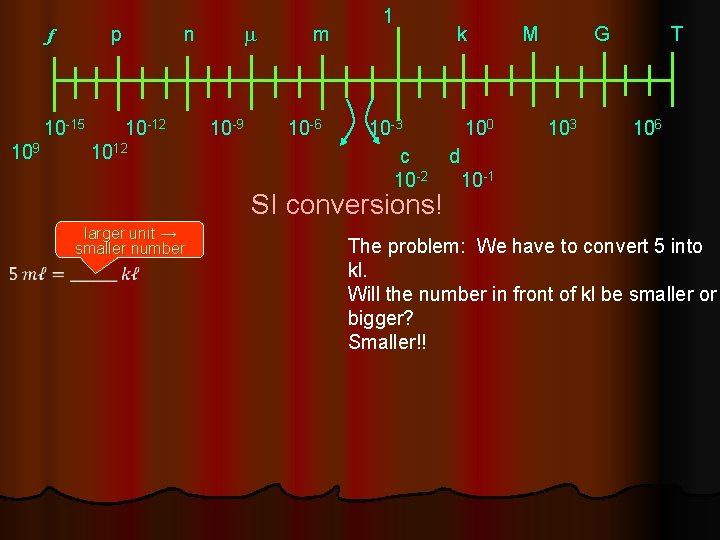
base unit 1 femto pico nano micro mili kilo mega giga tera f p n m m k M G T 10 -15 10 -12 10 -9 10 -6 10 -3 100 103 106 9 1012 10 centi deci c d Larger units Smaller units 10 -2 10 -1 every step is 10± 1 power They are grouped into steps 10± 3

f p n m 1 m k M G T 10 -15 10 -12 10 -9 10 -6 10 -3 100 103 106 9 1012 10 c d 10 -2 10 -1 SI conversions! larger unit → smaller number The problem: We have to convert 5 into kl. Will the number in front of kl be smaller or bigger? Smaller!!

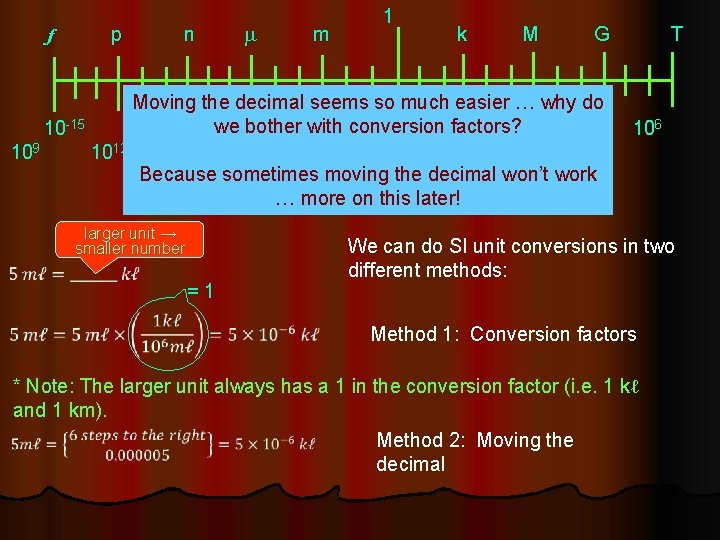
f p n m 1 m k M G T Moving the decimal seems so much easier … why do -9 10 -6 10 -3 100 103 106 we bother with conversion factors? 10 -15 10 -12 10 9 1012 10 c d Because sometimes moving the decimal won’t work -2 10 -1 10 … more on this later! SI conversions! larger unit → smaller number = 1 We can do SI unit conversions in two different methods: Method 1: Conversion factors * Note: The larger unit always has a 1 in the conversion factor (i. e. 1 kℓ and 1 km). Method 2: Moving the decimal

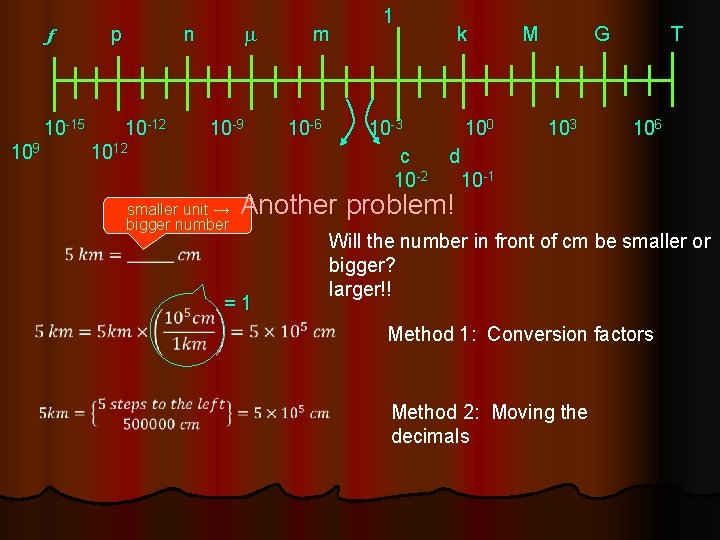
f p n m 1 m k M G T 10 -15 10 -12 10 -9 10 -6 10 -3 100 103 106 9 1012 10 c d 10 -2 10 -1 smaller unit → bigger number Another problem! = 1 Will the number in front of cm be smaller or bigger? larger!! Method 1: Conversion factors Method 2: Moving the decimals

One more as a class … The wavelength of green light is 500 nm. How many meters is this? or

Practice using both methods on your whiteboard … hold it up when you are done I have 906 gigabyte hard drive on my computer. How many bytes of data will it hold? 9. 06 X 1010 bytes Now practice more. If you are confident, do them by yourself. If you are not confident, then work with someone who is confident. BUT, the less confident one should be the one leading the practice and talking out their process!!!!

Practice 1: Basic SI conversions l

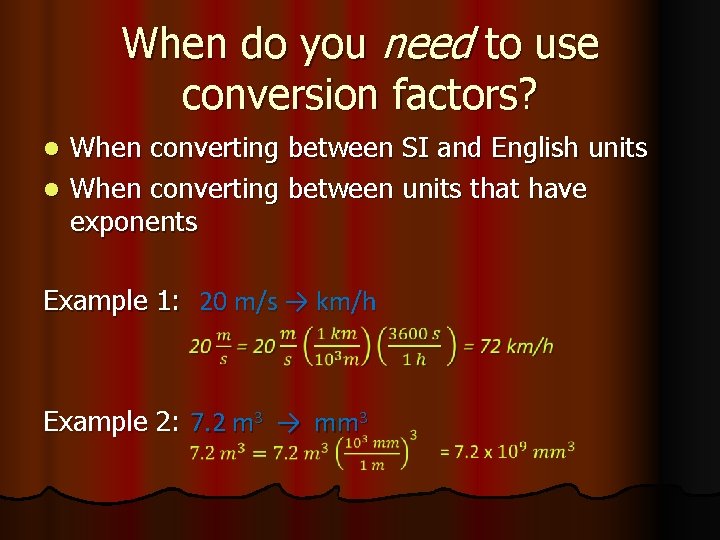
When do you need to use conversion factors? When converting between SI and English units l When converting between units that have exponents l Example 1: 20 m/s → km/h Example 2: 7. 2 m 3 → mm 3

Let’s do two as a class … Problem 1: 100 mm 3 → m 3 Problem 2: 60 mi/h = ? m/s = 10 -7 m 3 HINT: 1 mi = 1609 m

Practice on your whiteboard … hold it up when you are done 75 g/cm 2 → kg/m 2 Now practice more (use homework). . If you are confident, do them by yourself. If you are not confident, then work with someone who is confident. BUT, the less confident one should be the one leading the practice and talking out their process!!!!

Closure l What were our objectives today and how did we meet them? l How did what we do today reflect our statement of inquiry? l What was our learner profile trait, and how did we demonstrate it?

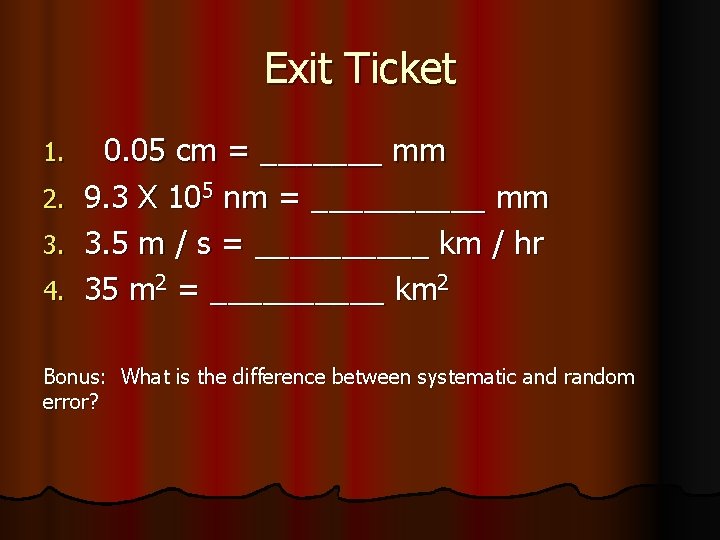
Exit Ticket 1. 2. 3. 4. 0. 05 cm = _______ mm 9. 3 X 105 nm = _____ mm 3. 5 m / s = _____ km / hr 35 m 2 = _____ km 2 Bonus: What is the difference between systematic and random error?