Dilution Problems 2 step Molarity Problems Study them

Dilution Problems

2 -step Molarity Problems • Study them because that’s the process we need to go through if we are making a solution from a solid solute. • For example, if I want a 1 M Na. Cl solution, I have to weigh out 1 mole or 58. 5 grams of Na. Cl and add water until I have 1 Liter of solution.

Dilution Problems • Sometimes we make a dilute solution from a very concentrated solution.

Dilution & Evaporation • Amount of solvent changes. • Amount of solute is constant.
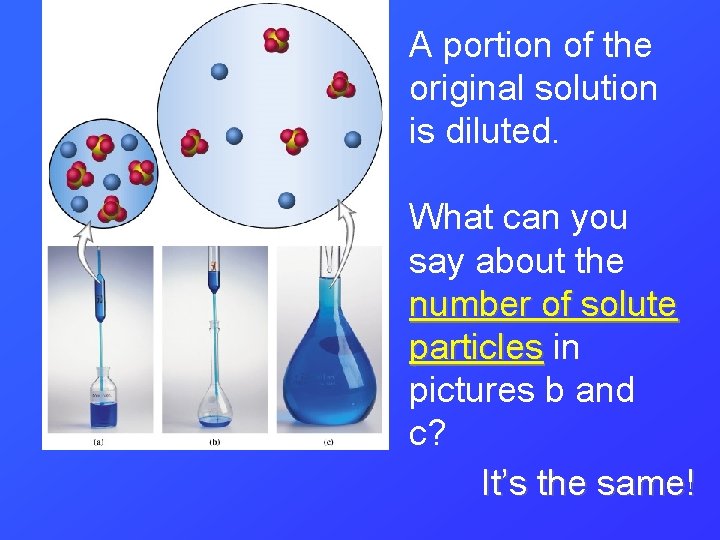
A portion of the original solution is diluted. What can you say about the number of solute particles in pictures b and c? It’s the same!
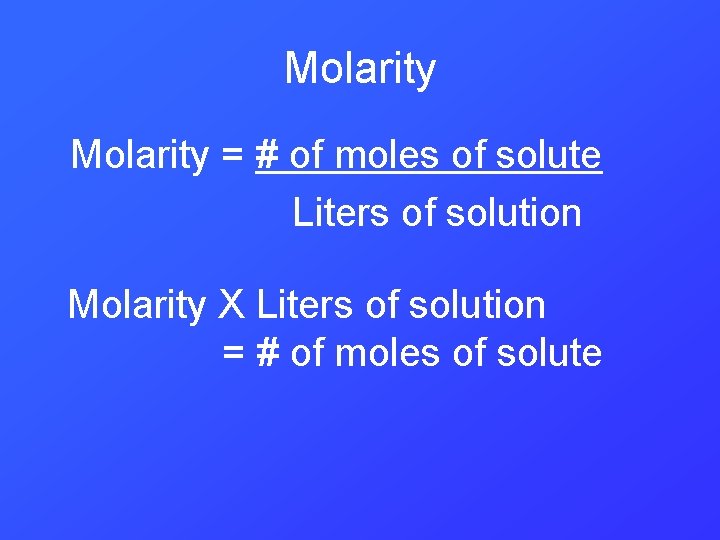
Molarity = # of moles of solute Liters of solution Molarity X Liters of solution = # of moles of solute

Amount of solute is constant Amount of solute in concentrated solution = Amount of solute in dilute solution
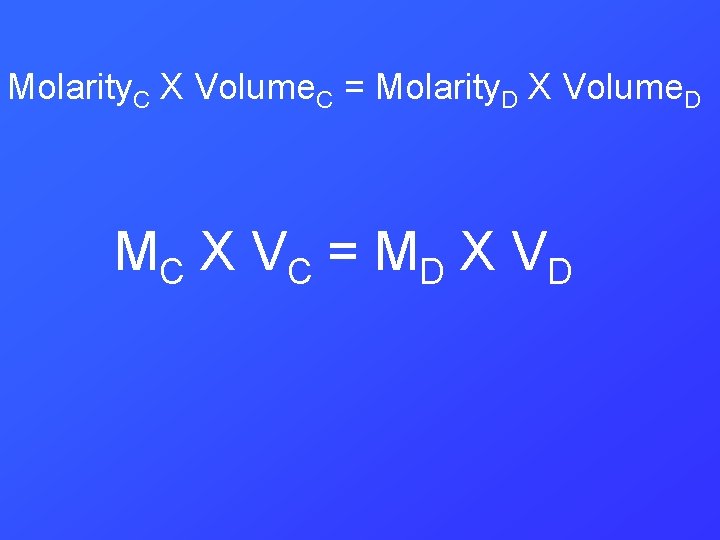
Molarity. C X Volume. C = Molarity. D X Volume. D MC X V C = M D X V D

Dilution problems • Important to pair up the molarity and volumes correctly. • The more concentrated molarity is the larger number. The dilute molarity is the smaller number.

How much 0. 25 M Na. Cl solution can be made from 1. 0 Liter of 1. 0 M Na. Cl solution? • Identify the variables • Plug into equation • • • (0. 25)X = (1. 0) • 0. 25 X =1. 0 • X = 4. 0 MD = 0. 25 VD = X MC = 1. 0 M VC = 1. 0 Liter 4. 0 Liters of 0. 25 M Na. Cl can be made.

Dilution problems • Twist: Sometimes they want to know how much water you have to add to the concentrated solution to get the dilute solution. • The dilution formula gives the volume of the dilute solution and the volume of the concentrated solution, NOT the volume of water. • VD = VC + VH 2 O so VD – VC = VH 2 O

How much water must be added to 0. 5 Liters of 3. 0 M Na. Cl to make 1. 0 M Na. Cl? • Identify Variables • Plug into equation • • • (1. 0)X = (3. 0)(0. 5) • X = 1. 5 Liters = VD MC = 3. 0 M VC = 0. 5 Liters MD = 1. 0 M VD = X • That’s NOT how much water you need to add. That’s the final volume!

Finding the water you add… • End up with 1. 5 liters of dilute solution. • Started with 0. 5 liters of concentrated solution. • The difference, 1. 0 liter, is how much water must be added.
- Slides: 13