Dilution and Spectroscopy Lab Word Document Dilution Background
Dilution and Spectroscopy Lab Word Document

Dilution Background • Acids and bases usually come in concentrated form, as “concentrates, ” but they are rarely used in this form. • A dilution is prepared by adding a specific amount of a concentrate to water to obtain a new volume and concentration. • In order to calculate dilutions of solutions the equation: M 1 V 1 = M 2 V 2 where M is concentration in molarity (mol solute/L solution) and V is volume (L), is used

Dilution Examples • Dilutions by a factor of 2: – Take 3 m. L of “acid” and dilute with 3 m. L of water – 1 part concentrate, 1 part water • Dilutions by a factor of 4: – 1 part concentrate, 3 parts water • Dilutions by a factor of 10: – 1 part concentrate, 9 parts water
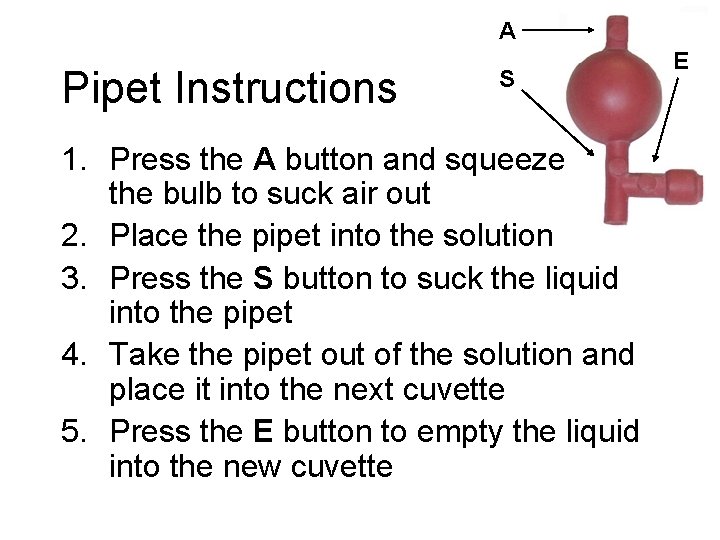
A Pipet Instructions S 1. Press the A button and squeeze the bulb to suck air out 2. Place the pipet into the solution 3. Press the S button to suck the liquid into the pipet 4. Take the pipet out of the solution and place it into the next cuvette 5. Press the E button to empty the liquid into the new cuvette E

Spectroscopy Introduction • Spectroscopy is one method of determining the concentration of an unknown solution. • By measuring the respective absorbance values for solutions with known concentrations, a calibration curve can be constructed. • The absorbance of an unknown can be used to determine its concentration through use of this calibration curve.
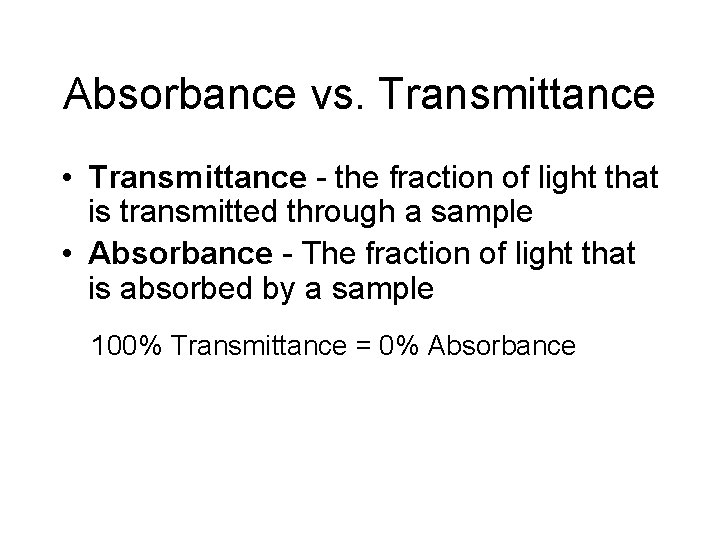
Absorbance vs. Transmittance • Transmittance - the fraction of light that is transmitted through a sample • Absorbance - The fraction of light that is absorbed by a sample 100% Transmittance = 0% Absorbance
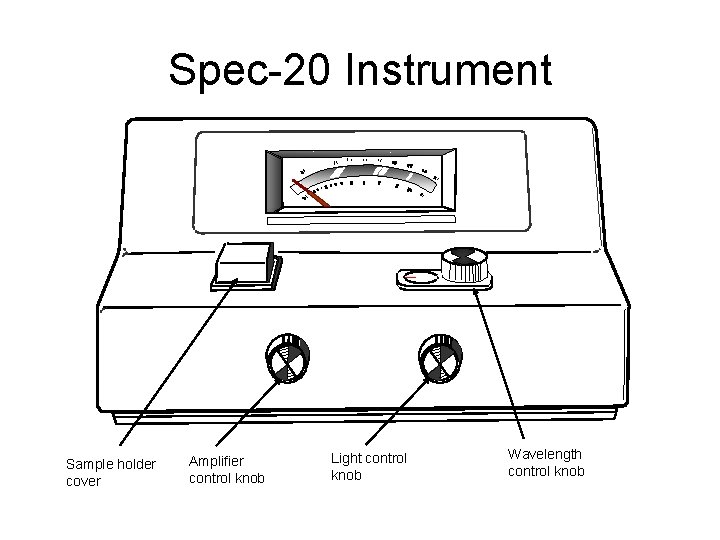
Spec-20 Instrument Sample holder cover Amplifier control knob Light control knob Wavelength control knob
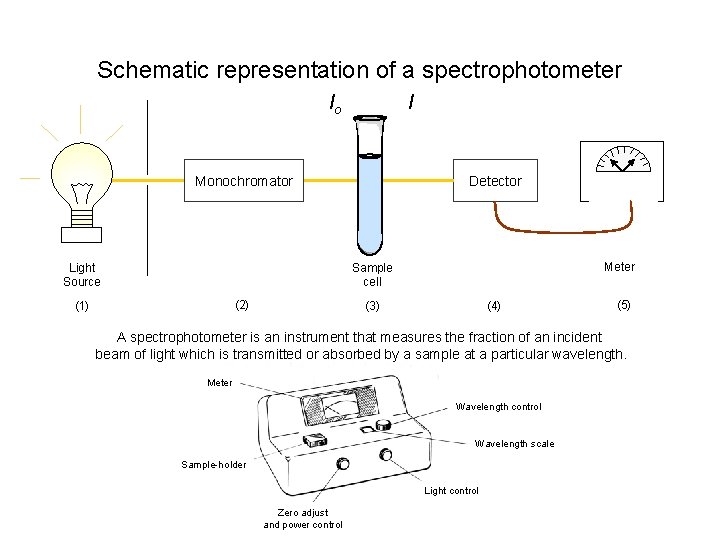
Schematic representation of a spectrophotometer I Io Monochromator Light Source Detector Meter Sample cell (2) (1) (4) (3) (5) A spectrophotometer is an instrument that measures the fraction of an incident beam of light which is transmitted or absorbed by a sample at a particular wavelength. Meter Wavelength control Wavelength scale Sample-holder Light control Zero adjust and power control
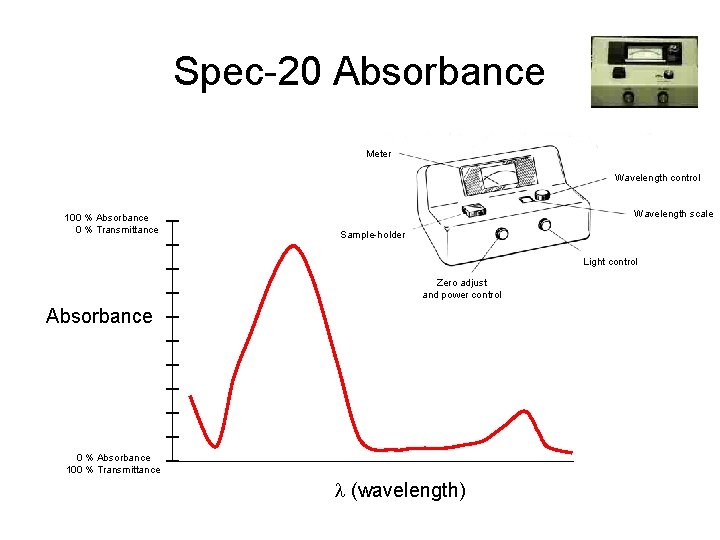
Spec-20 Absorbance Insert Photograph Spec-20 Meter Wavelength control 100 % Absorbance 0 % Transmittance Wavelength scale Sample-holder Light control Zero adjust and power control Absorbance 0 % Absorbance 100 % Transmittance l (wavelength)
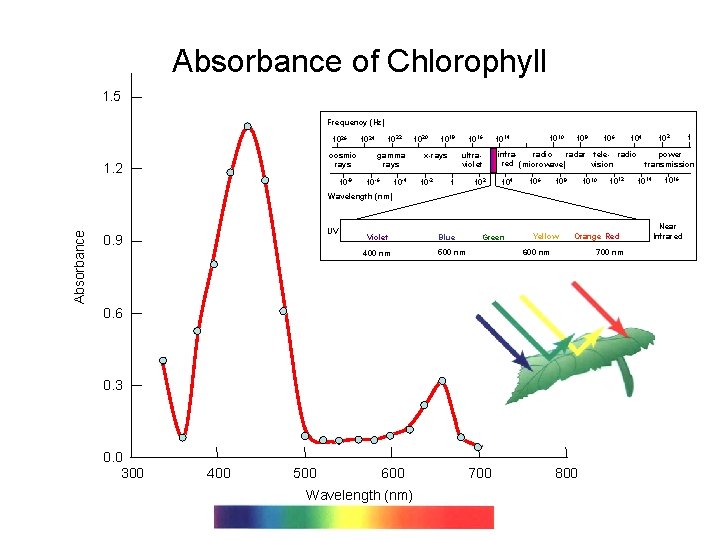
Absorbance of Chlorophyll 1. 5 Frequency (Hz) 1026 cosmic rays 1. 2 10 -8 1022 1024 gamma rays 10 -4 10 -6 1020 1018 x-rays 10 -2 1016 ultraviolet 1 102 1010 1014 106 108 infraradio radar tele- radio red (microwave) vision 104 106 108 1010 1012 104 power transmission 1014 1016 Absorbance Wavelength (nm) UV 0. 9 Violet 400 nm Blue Green Yellow Orange Red 700 nm 600 nm 500 nm 0. 6 0. 3 0. 0 300 400 500 600 Wavelength (nm) 700 800 1 Near Infrared
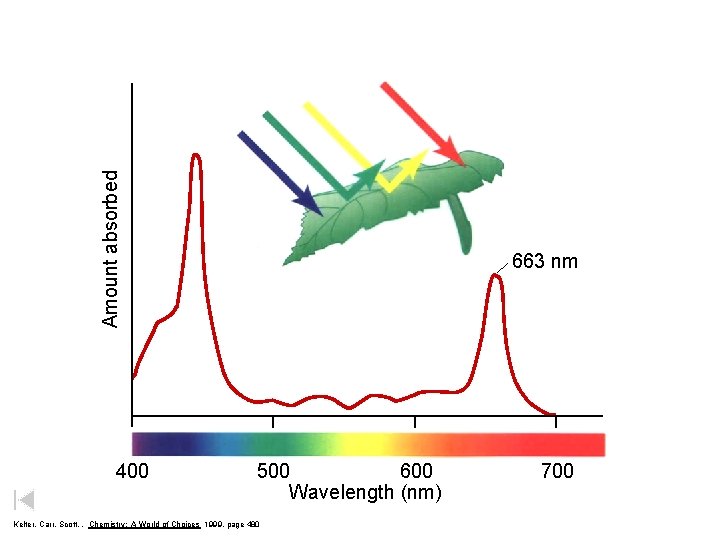
Amount absorbed 400 663 nm 500 600 Wavelength (nm) Kelter, Carr, Scott, , Chemistry: A World of Choices 1999, page 480 700
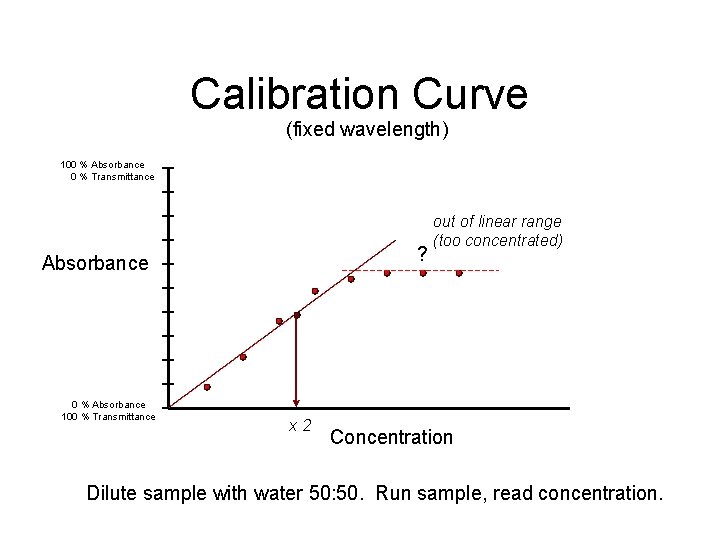
Calibration Curve (fixed wavelength) 100 % Absorbance 0 % Transmittance ? Absorbance 0 % Absorbance 100 % Transmittance x 2 out of linear range (too concentrated) Concentration Dilute sample with water 50: 50. Run sample, read concentration.
Dilution of Solutions Dilution of Solutions Keys http: //www. unit 5. org/chemistry/Solutions. html
- Slides: 13