Dilute vs Concentrated Concentrated solutions contain a high






- Slides: 17

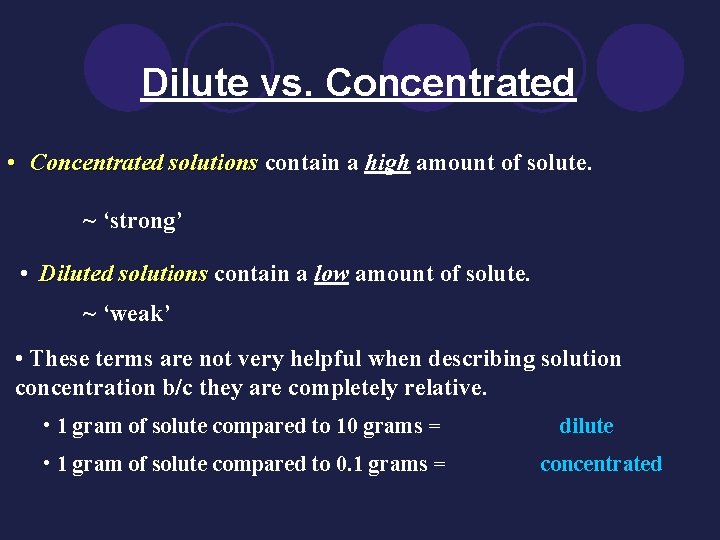
Dilute vs. Concentrated • Concentrated solutions contain a high amount of solute. ~ ‘strong’ • Diluted solutions contain a low amount of solute. ~ ‘weak’ • These terms are not very helpful when describing solution concentration b/c they are completely relative. • 1 gram of solute compared to 10 grams = • 1 gram of solute compared to 0. 1 grams = dilute concentrated

Types of Solutions • Unsaturated solutions contain a less solute than they are capable of dissolving at a given T and P. ~ can hold more…not full yet! • Saturated solutions contain the max. amount of solute that they are capable of dissolving at a given T and P. ~ equilibrium exists b/n dissolved and undissolved solute. ~ completely full, can’t hold any more! • Supersaturated solutions contain more solute (dissolved) than it is supposed to hold at a given T and P. ~ must heat solution up to allow more solute to dissolve ~ then let it cool down very slowly, undisturbed.

Solution Calculations • we said that ‘dilute’ and ‘concentrated’ are very general terms for solution concentration. • Molarity indicates how many moles of solute are dissolved in one liter of solution. ~ molarity = moles Liters ~ units = M • Ex… What is the molarity of a salt water solution containing 9. 0 moles of salt dissolved in 3. 0 liters of solution? 9. 0 moles 3. 0 liters = 3. 0 M

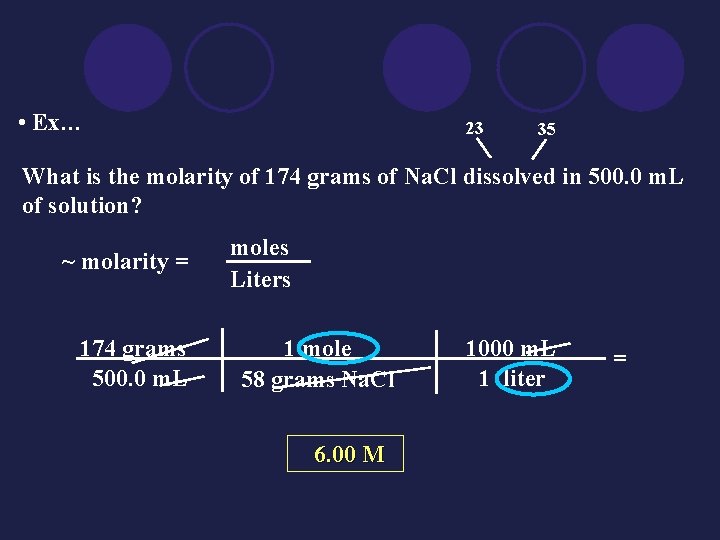
• Ex… 23 35 What is the molarity of 174 grams of Na. Cl dissolved in 500. 0 m. L of solution? ~ molarity = 174 grams 500. 0 m. L moles Liters 1 mole 58 grams Na. Cl 6. 00 M 1000 m. L 1 liter =

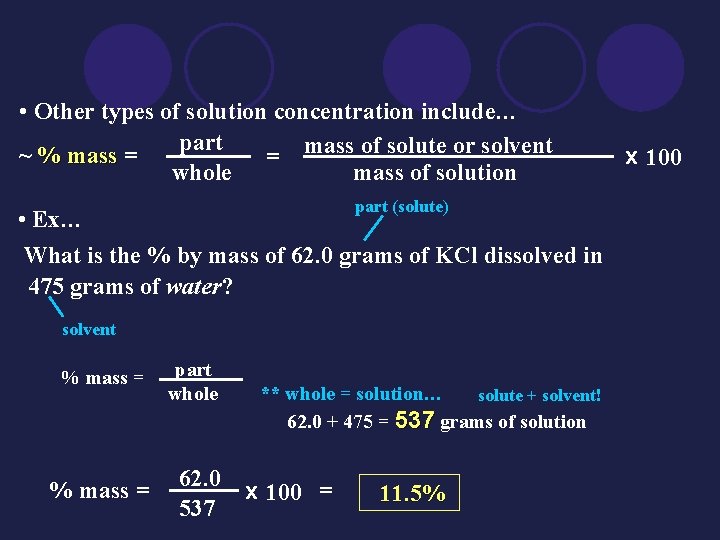
• Other types of solution concentration include… part ~ % mass = = mass of solute or solvent whole mass of solution part (solute) • Ex… What is the % by mass of 62. 0 grams of KCl dissolved in 475 grams of water? solvent % mass = part whole % mass = 62. 0 537 ** whole = solution… solute + solvent! 62. 0 + 475 = 537 grams of solution x 100 = 11. 5% x 100

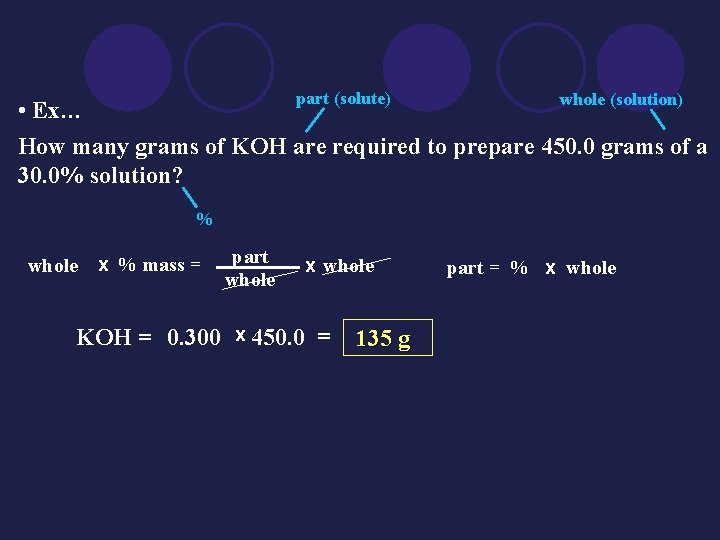
part (solute) whole (solution) • Ex… How many grams of KOH are required to prepare 450. 0 grams of a 30. 0% solution? % whole x % mass = part whole x whole KOH = 0. 300 x 450. 0 = 135 g part = % x whole

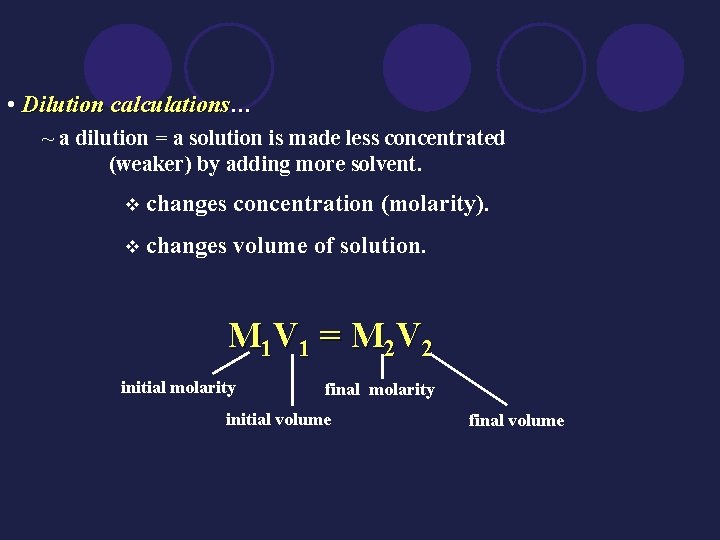
• Dilution calculations… ~ a dilution = a solution is made less concentrated (weaker) by adding more solvent. v changes concentration (molarity). v changes volume of solution. M 1 V 1 = M 2 V 2 initial molarity final molarity initial volume final volume

• Ex… V 1 M 1 • How many liters of a 12 M solution are needed to create 2. 0 liters of a 4. 0 M solution? V 2 M 2 12 X = 2. 0 (4. 0) • Ex… M 1 V 1 = M 2 V 2 12 X = 8. 0 12 12 M 1 0. 67 liters X= V 1 • What is the molarity of 1. 5 liters of solution made from 600. 0 m. L of 10. 0 M Na. OH? V 2 M 2 1. 5 X = 0. 6000 (10. 0) 1. 5 X = 6. 00 1. 5 X= 4. 0 M

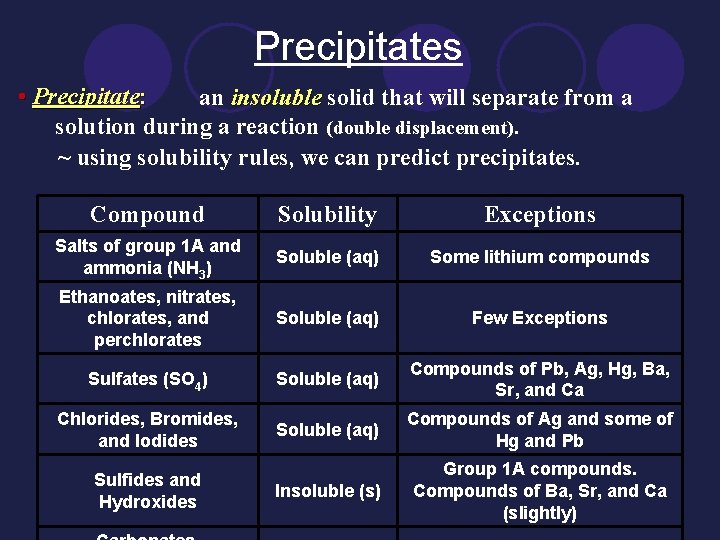
Precipitates • Precipitate: an insoluble solid that will separate from a solution during a reaction (double displacement). ~ using solubility rules, we can predict precipitates. Compound Solubility Exceptions Salts of group 1 A and ammonia (NH 3) Soluble (aq) Some lithium compounds Ethanoates, nitrates, chlorates, and perchlorates Soluble (aq) Few Exceptions Sulfates (SO 4) Soluble (aq) Compounds of Pb, Ag, Hg, Ba, Sr, and Ca Chlorides, Bromides, and Iodides Soluble (aq) Compounds of Ag and some of Hg and Pb Insoluble (s) Group 1 A compounds. Compounds of Ba, Sr, and Ca (slightly) Sulfides and Hydroxides

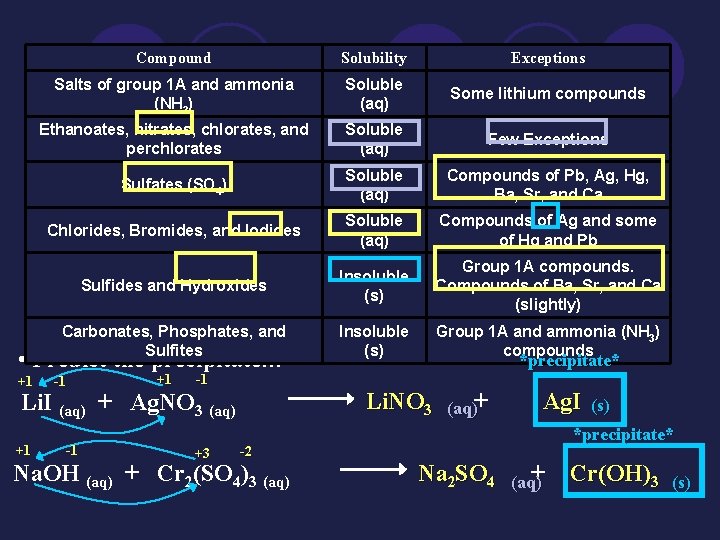
Compound Solubility Exceptions Salts of group 1 A and ammonia (NH 3) Soluble (aq) Some lithium compounds Ethanoates, nitrates, chlorates, and perchlorates Soluble (aq) Few Exceptions Sulfates (SO 4) Soluble (aq) Compounds of Pb, Ag, Hg, Ba, Sr, and Ca Chlorides, Bromides, and Iodides Soluble (aq) Compounds of Ag and some of Hg and Pb Sulfides and Hydroxides Insoluble (s) Group 1 A compounds. Compounds of Ba, Sr, and Ca (slightly) Carbonates, Phosphates, and Sulfites Insoluble (s) Group 1 A and ammonia (NH 3) compounds • Predict the precipitate… +1 -1 Li. NO 3 Li. I (aq) + Ag. NO 3 (aq) +1 -1 +3 *precipitate* -2 Na. OH (aq) + Cr 2(SO 4)3 (aq)+ Ag. I (s) *precipitate* Na 2 SO 4 (aq+) Cr(OH)3 (s)

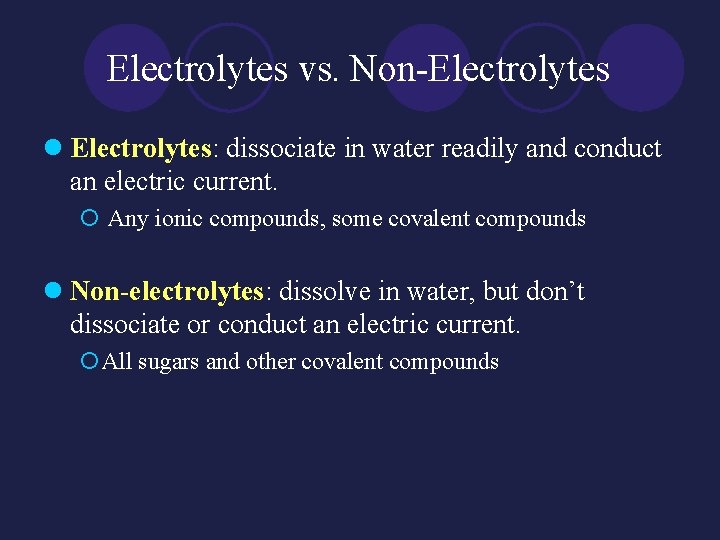
Electrolytes vs. Non-Electrolytes l Electrolytes: dissociate in water readily and conduct an electric current. ¡ Any ionic compounds, some covalent compounds l Non-electrolytes: dissolve in water, but don’t dissociate or conduct an electric current. ¡All sugars and other covalent compounds

Colligative Properties • Colligative properties are properties of solutions that are affected only by the # of particles in the solution. • NOT affected by the type of particle!!! • Ex… • ~ vapor pressure (VP) • ~ freezing point (FP) • ~ boiling point (BP)

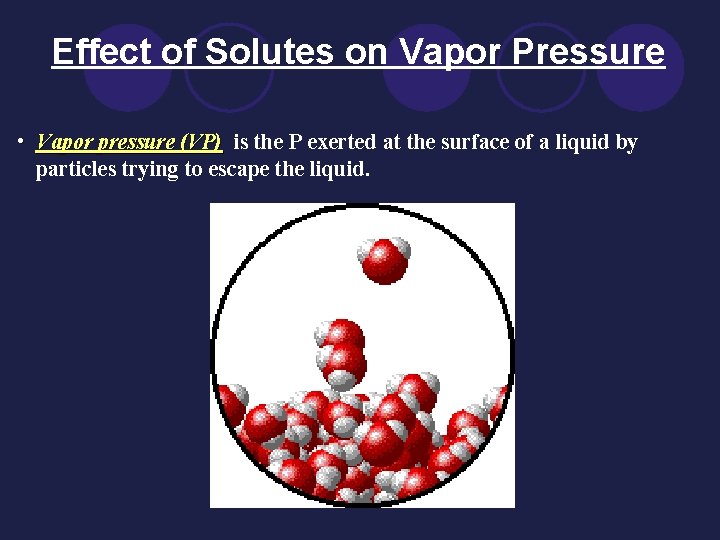
Effect of Solutes on Vapor Pressure • Vapor pressure (VP) is the P exerted at the surface of a liquid by particles trying to escape the liquid.

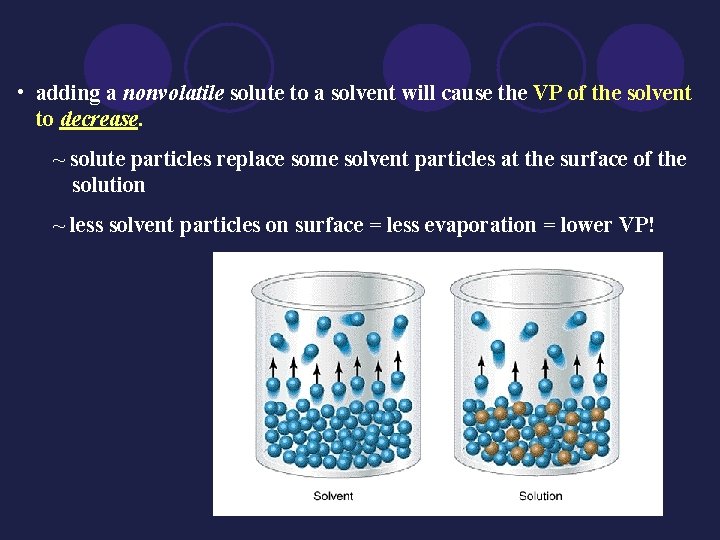
• adding a nonvolatile solute to a solvent will cause the VP of the solvent to decrease. ~ solute particles replace some solvent particles at the surface of the solution ~ less solvent particles on surface = less evaporation = lower VP!

How Solutes Affect BP and FP • Boiling Point (BP) is temp. at which the VP of the liquid = atmospheric pressure. ~ adding solute lowers VP of solvent ~ must add more KE (heat) to equalize the pressures ** solutes RAISE the BP of solutions! (i. e. we add salt before we boil water) pure water salt water

• Freezing Point (FP) is temp. at which liquid turns into a solid. ~ enough KE is lost (removal of heat) that molecules stop moving around and lock into place. ~ adding solute lowers VP of solvent ~ even more KE (heat) must be lost to lock molecules into place. ** solutes LOWER the FP of solutions! (i. e. we add salt to icy roads…salt is used in making ice cream )

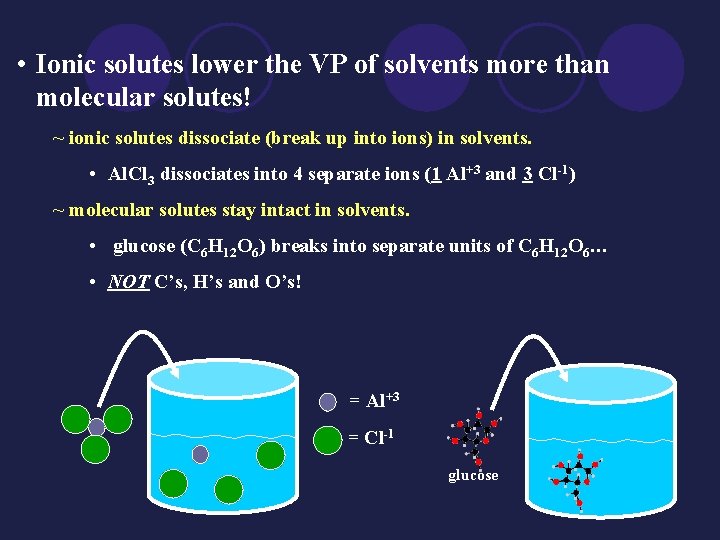
• Ionic solutes lower the VP of solvents more than molecular solutes! ~ ionic solutes dissociate (break up into ions) in solvents. • Al. Cl 3 dissociates into 4 separate ions (1 Al+3 and 3 Cl-1) ~ molecular solutes stay intact in solvents. • glucose (C 6 H 12 O 6) breaks into separate units of C 6 H 12 O 6… • NOT C’s, H’s and O’s! = Al+3 = Cl-1 glucose