Dihydrogen Monoxide Why Chemistry Joke If H 20






















![FLASHBACK EOC WORKBOOK Pg. 43 [all] FLASHBACK EOC WORKBOOK Pg. 43 [all]](https://slidetodoc.com/presentation_image_h2/aa3a37cd9d0f0cdee545a2d50f42e33a/image-33.jpg)





![THURSDAY 10/22 - BELLRINGER EOC WORKBOOK Pg. 45 [# 2 -6] Pg. 46 [# THURSDAY 10/22 - BELLRINGER EOC WORKBOOK Pg. 45 [# 2 -6] Pg. 46 [#](https://slidetodoc.com/presentation_image_h2/aa3a37cd9d0f0cdee545a2d50f42e33a/image-47.jpg)


- Slides: 49

Dihydrogen Monoxide!!!! Why? ?

Chemistry Joke! If H 20 is water, what is H 204? Drinking, bathing, washing, swimming… All kinds of things!

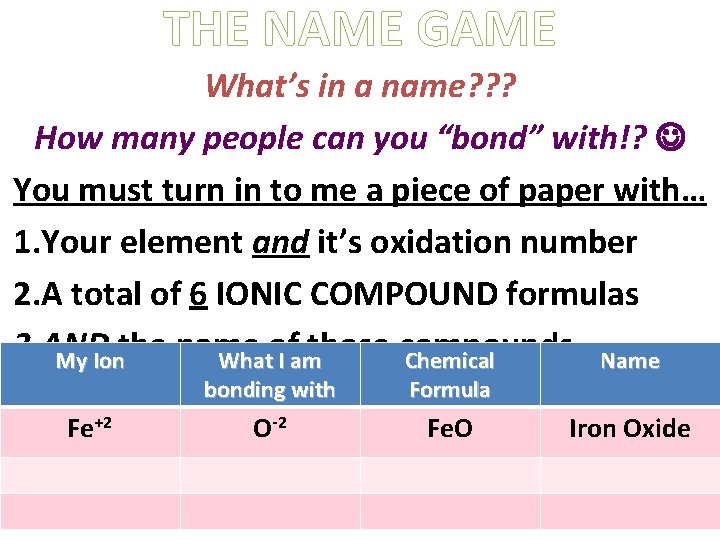
THE NAME GAME What’s in a name? ? ? How many people can you “bond” with!? You must turn in to me a piece of paper with… 1. Your element and it’s oxidation number 2. A total of 6 IONIC COMPOUND formulas 3. AND those compounds My Ionthe name Whatof I am Chemical Name Fe+2 bonding with Formula O-2 Fe. O Iron Oxide

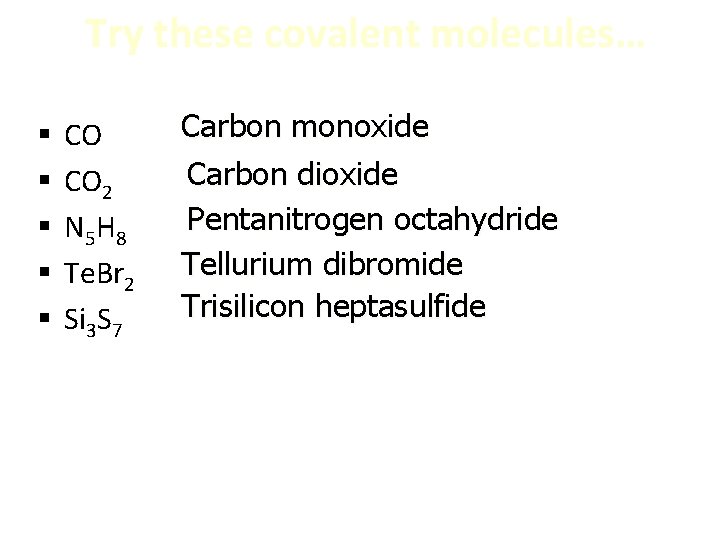
Try these covalent molecules… § § § CO CO 2 N 5 H 8 Te. Br 2 Si 3 S 7 Carbon monoxide Carbon dioxide Pentanitrogen octahydride Tellurium dibromide Trisilicon heptasulfide

FLASHBACK 1. How many oxygen atoms are there in bleach, Na. Cl. O, AKA sodium hypochlorite? 2. Acetone (CH 3 COCH 3), or nail polish remover, has how many total hydrogen atoms? 3. How do you determine oxidation #’s? 4. What is the oxidation # of Al, O, & Cl ? 5. Write ionic formulas: (find charges 1 st then criss-cross!!) Mg + F Be + N 6. Name the following compounds: N 5 H 8 Sr. Cl 2

MORE NAMING PRACTICE • COVALENT: –NO 2 –N 2 O • IONIC: –Na. F –Zn. Cl

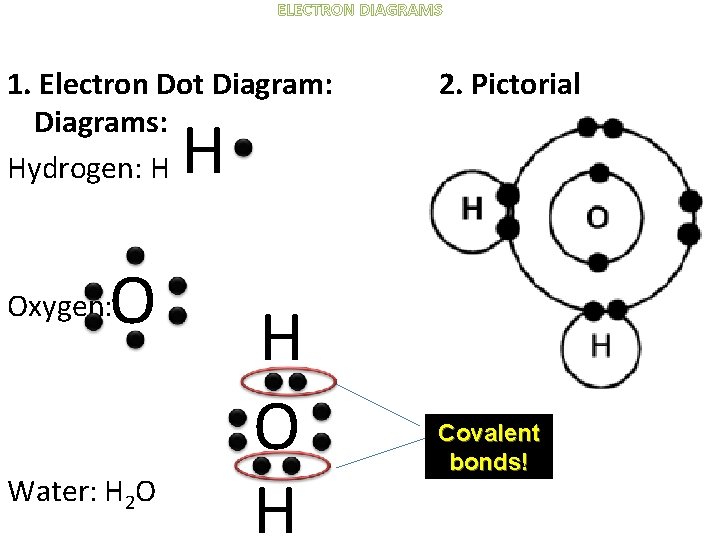
ELECTRON DIAGRAMS 1. Electron Dot Diagram: Diagrams: Hydrogen: H 2. Pictorial H O Oxygen: Water: H 2 O H Covalent bonds!

Chemical Changes and Chemical Reactions

PHYSICAL CHANGES Occur when the size or shape of the substance is changed Occasionally, the color can change, too Regardless, the original substance(s) do not change Evidences of Physical Changes: - Bending, stretching, heat, and cooling can all cause a physical change ***All phase changes are physical changes

CHEMICAL CHANGES Occurs when there is a change in the arrangement of atoms so that a different substance with different properties is produced Very often, there is some kind of evidence (for example, the formation

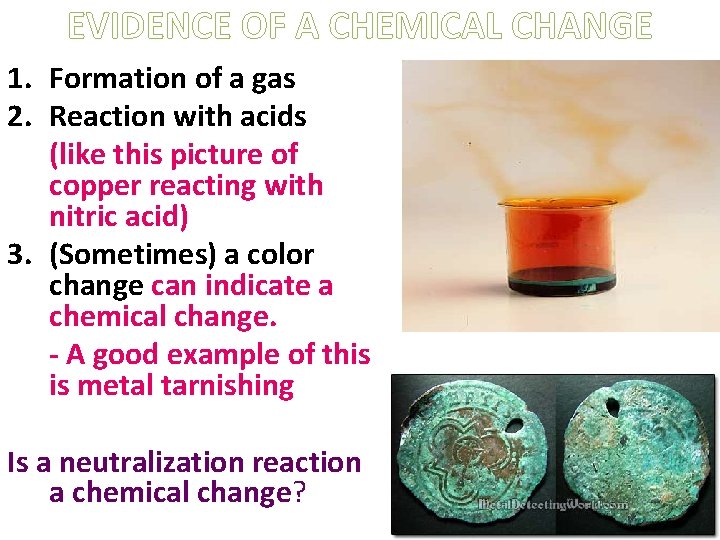
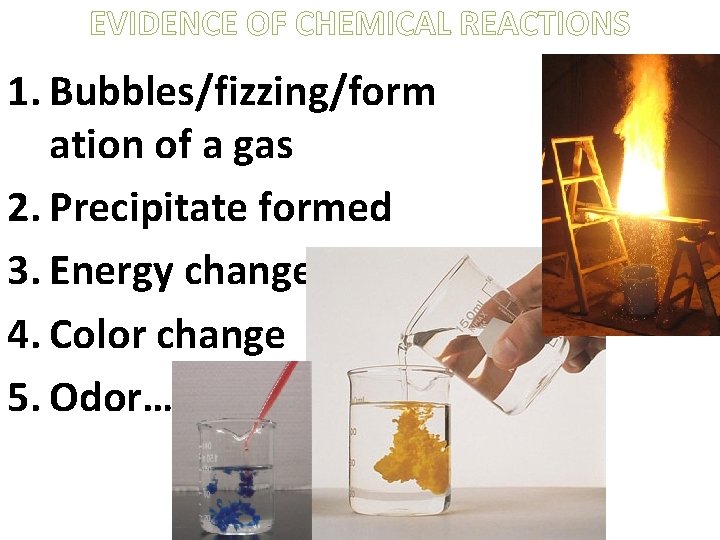
EVIDENCE OF A CHEMICAL CHANGE 1. Formation of a gas 2. Reaction with acids (like this picture of copper reacting with nitric acid) 3. (Sometimes) a color change can indicate a chemical change. - A good example of this is metal tarnishing Is a neutralization reaction a chemical change?

EVIDENCE OF CHEMICAL REACTIONS 1. Bubbles/fizzing/form ation of a gas 2. Precipitate formed 3. Energy change 4. Color change 5. Odor…

Chemistry Joke! If you're not part of the solution… You're part of the precipitate!

FLASHBACK 1. What is the main difference between a chemical and physical change? 2. If a reaction forms a gas, you know it is a _______ change. 3. If something changes color, you know it’s a chemical change. True False 4. Name the four evidences of a chemical rxn. 5. Explain a situation in which bubbling occurs, but it is NOT a chemical change.

Why do you burp after drinking a Coke?

Excuse me… • Coke and other soft drinks are carbonated • Carbonation occurs when carbon dioxide is dissolved in water or solution • This gives the "fizz" to carbonated beverages • Excess gas needs out of the stomach. . So we burp!

ROCKET LAB TIME!! Using the materials provided: 1. 2 pieces of Alka-Seltzer 2. 1 film canister 3. Water …you are to build a projectile!

HYPOTHESIZING Pick ONE question to answer and generate a hypothesis and WRITE IT DOWN… Use an “If-Then” statement! 1. How does changing the volume of water effect the time/height of rocket “launch”? 2. How does changing the amount of Alka. Seltzer effect the time/height of rocket

Lab Report You are to write a brief lab report on your experience… - Skip lines between headings - Full sentences! Alka seltzer Title Hypothesis: (your question) Data: Table? List? Conclusion: tell me what you learned (in paragraph form!) 1. What evidence did you see of a chemical reaction taking place? How does this relate to the lab? 2. Refer back to your hypothesis… was it right or wrong? ? Why? ? 3. What would have done differently… or how would

FLASHBACK EOC WORKBOOK Pg. 41 (all) A. An exothermic reaction _________ heat. B. An endothermic reaction _________ heat. C. ( A + B AB ) is an example of a _______ reaction

Naming: 2)P 2 O 5 ______________ 3)Mg 3 N ______________ 5)Si. O 2 ______________ 6)Ba. Cl 2 ________________ 8)B 2 P 9 ___________________ Formulas: 14) aluminum nitride __________ 16) disulfur pentaphosphide __________ 17) potassium sulfide _____________

Chemical Reactions and Equations: What do they mean? What do they show?

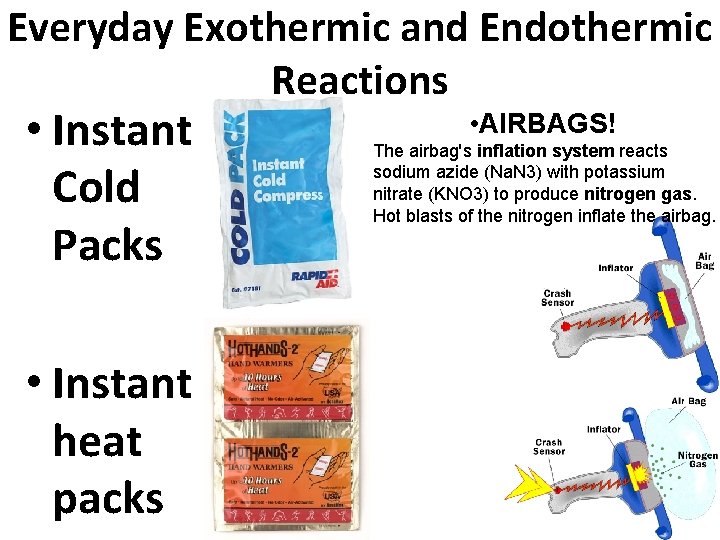
ENERGY CHANGES IN REACTIONS During any chemical reaction, there is an energy change. 1. Exothermic reaction: heat is released during the reaction, gets hot! 2. Endothermic reaction: heat is absorbed during the reaction,

Everyday Exothermic and Endothermic Reactions • Instant Cold Packs • Instant heat packs • AIRBAGS! The airbag's inflation system reacts sodium azide (Na. N 3) with potassium nitrate (KNO 3) to produce nitrogen gas. Hot blasts of the nitrogen inflate the airbag.

Videos • Exothermic vs. Endothermic • Endothermic Reaction

EQUATION TERMS A. Reactants: original substances entering a chemical rxn - what you started with, on the left side B. Products: resulting substances - what you end with, on the right side Reactants Products

Endothermic vs. Exothermic Calcium Chloride vs. Sodium Bicarbonate… who will win the temperature war? ? ? Turn into me: Half sheet of paper

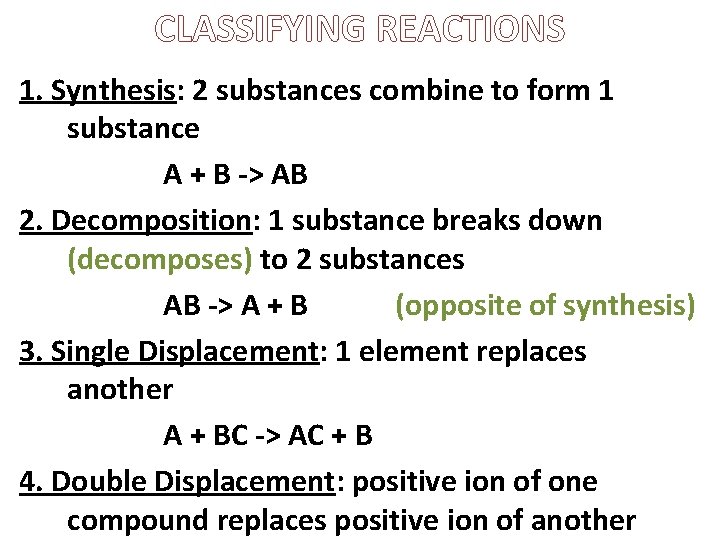
CLASSIFYING REACTIONS 1. Synthesis: 2 substances combine to form 1 substance A + B -> AB 2. Decomposition: 1 substance breaks down (decomposes) to 2 substances AB -> A + B (opposite of synthesis) 3. Single Displacement: 1 element replaces another A + BC -> AC + B 4. Double Displacement: positive ion of one compound replaces positive ion of another

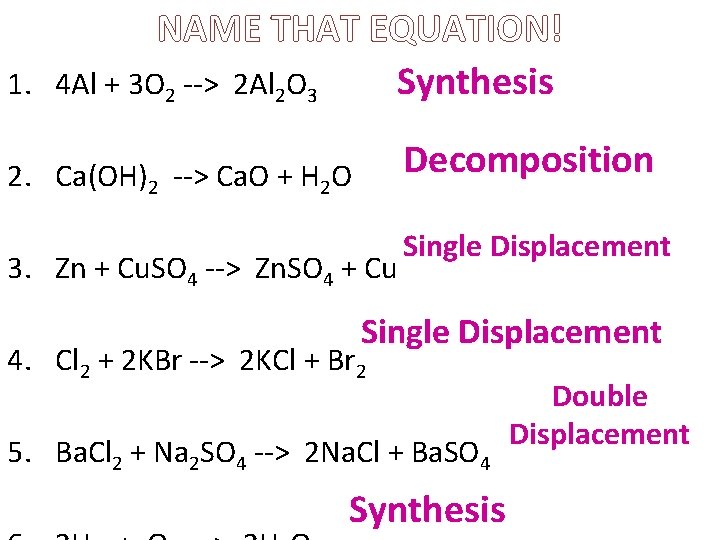
NAME THAT EQUATION! 1. 4 Al + 3 O 2 --> 2 Al 2 O 3 Synthesis Decomposition 2. Ca(OH)2 --> Ca. O + H 2 O 3. Zn + Cu. SO 4 --> Zn. SO 4 + Cu Single Displacement 4. Cl 2 + 2 KBr --> 2 KCl + Br 2 5. Ba. Cl 2 + Na 2 SO 4 --> 2 Na. Cl + Ba. SO 4 Synthesis Double Displacement

FLASHBACK Label the following equations: (4 types) 1. Zn + HCl Zn. Cl 2 + H 2 Single Displacement 2. Fe + O 2 Fe 2 O 3 Synthesis 3. Si. O 2 + HF Si. F 4 + H 2 ODouble Displacement 4. Fe. S + HCl H 2 S + Fe. Cl 2 5. In lab yesterday, what gas produced the signature “popping” sound?

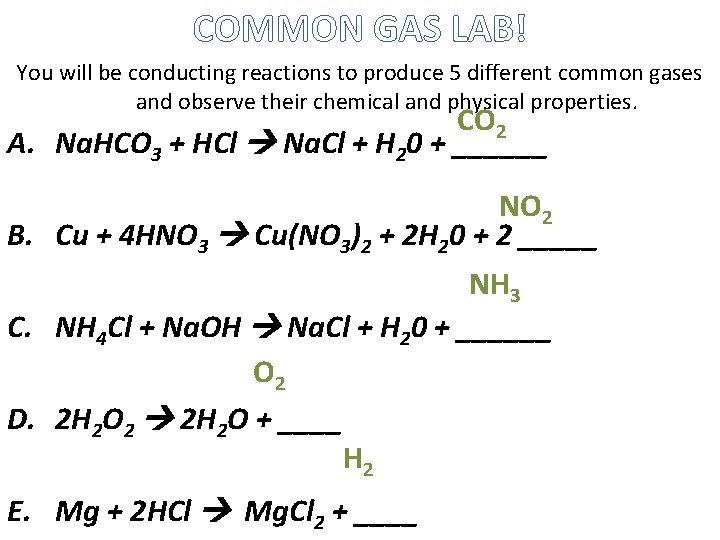
COMMON GAS LAB! You will be conducting reactions to produce 5 different common gases and observe their chemical and physical properties. CO 2 A. Na. HCO 3 + HCl Na. Cl + H 20 + ______ NO 2 B. Cu + 4 HNO 3 Cu(NO 3)2 + 2 H 20 + 2 _____ NH 3 C. NH 4 Cl + Na. OH Na. Cl + H 20 + ______ O 2 D. 2 H 2 O 2 2 H 2 O + ____ H 2 E. Mg + 2 HCl Mg. Cl 2 + ____

FLASHBACK Section Review Pg. 645 #1, 3 EOC REVIEW Pg. 656 #1 -5, 11, 15
![FLASHBACK EOC WORKBOOK Pg 43 all FLASHBACK EOC WORKBOOK Pg. 43 [all]](https://slidetodoc.com/presentation_image_h2/aa3a37cd9d0f0cdee545a2d50f42e33a/image-33.jpg)
FLASHBACK EOC WORKBOOK Pg. 43 [all]


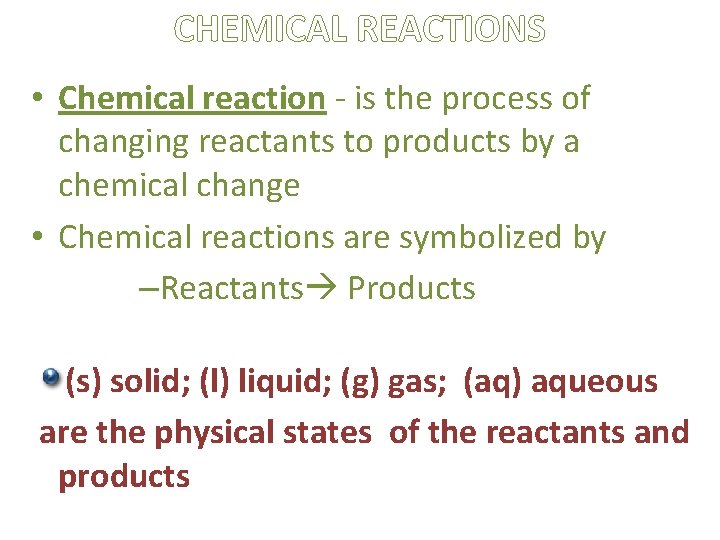
CHEMICAL REACTIONS • Chemical reaction - is the process of changing reactants to products by a chemical change • Chemical reactions are symbolized by –Reactants Products (s) solid; (l) liquid; (g) gas; (aq) aqueous are the physical states of the reactants and products

EQUATIONS SHOW… § The reactants which enter into a reaction. § The products which are formed by the reaction. § The amounts of each substance used and each substance produced. ___Mg 2 (s) + __ O 2(g) __ Mg. O(s) 2

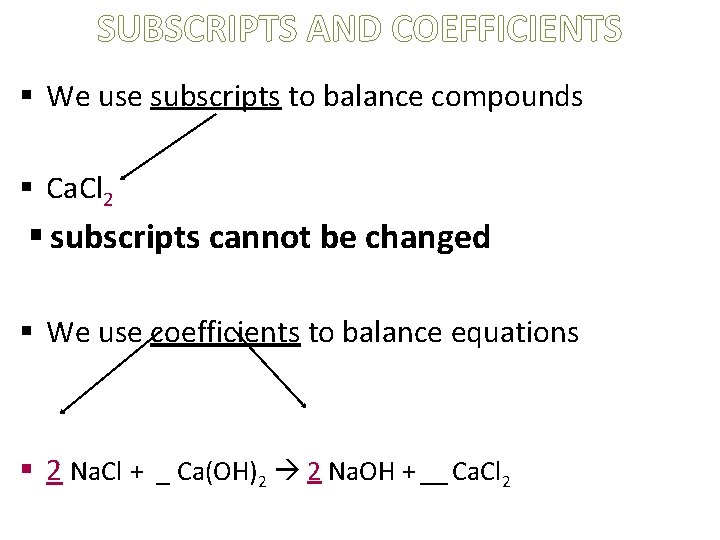
SUBSCRIPTS AND COEFFICIENTS § We use subscripts to balance compounds § Ca. Cl 2 § subscripts cannot be changed § We use coefficients to balance equations § 2 Na. Cl + _ Ca(OH)2 2 Na. OH + __ Ca. Cl 2

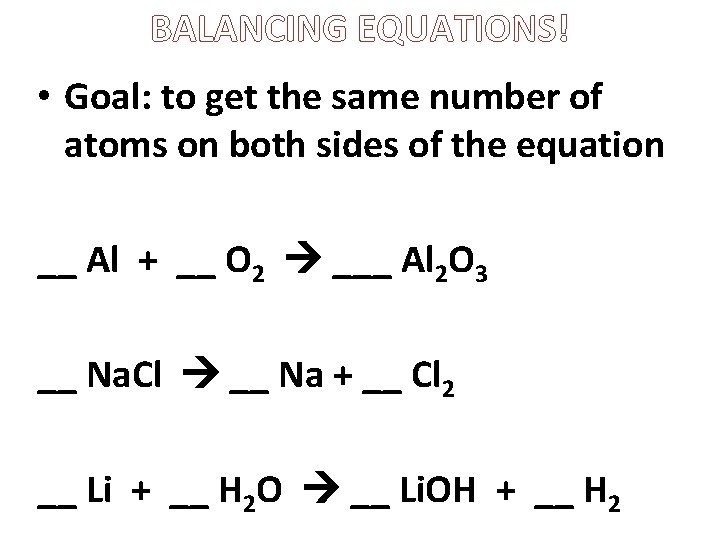
BALANCING EQUATIONS! • Goal: to get the same number of atoms on both sides of the equation __ Al + __ O 2 ___ Al 2 O 3 __ Na. Cl __ Na + __ Cl 2 __ Li + __ H 2 O __ Li. OH + __ H 2

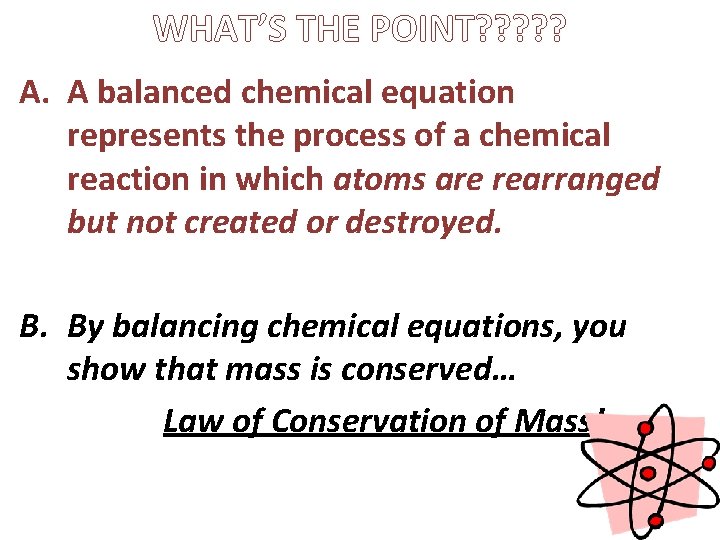
WHAT’S THE POINT? ? ? A. A balanced chemical equation represents the process of a chemical reaction in which atoms are rearranged but not created or destroyed. B. By balancing chemical equations, you show that mass is conserved… Law of Conservation of Mass!

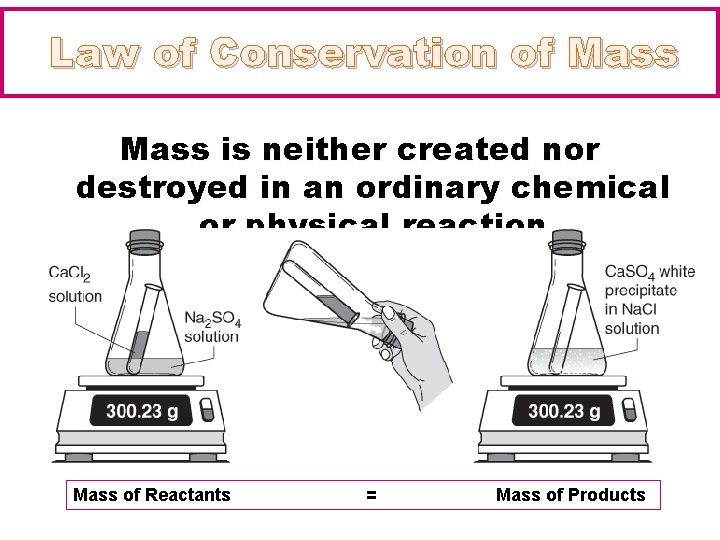
Law of Conservation of Mass is neither created nor destroyed in an ordinary chemical or physical reaction Mass of Reactants = Mass of Products

LAB: Looking at the… Law of Conservation of mass!!!

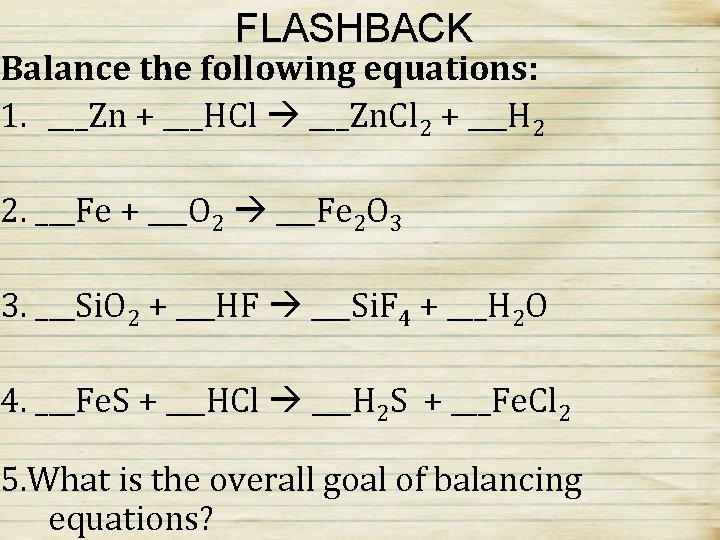
FLASHBACK Balance the following equations: 1. ___Zn + ___HCl ___Zn. Cl 2 + ___H 2 2. ___Fe + ___O 2 ___Fe 2 O 3 3. ___Si. O 2 + ___HF ___Si. F 4 + ___H 2 O 4. ___Fe. S + ___HCl ___H 2 S + ___Fe. Cl 2 5. What is the overall goal of balancing equations?

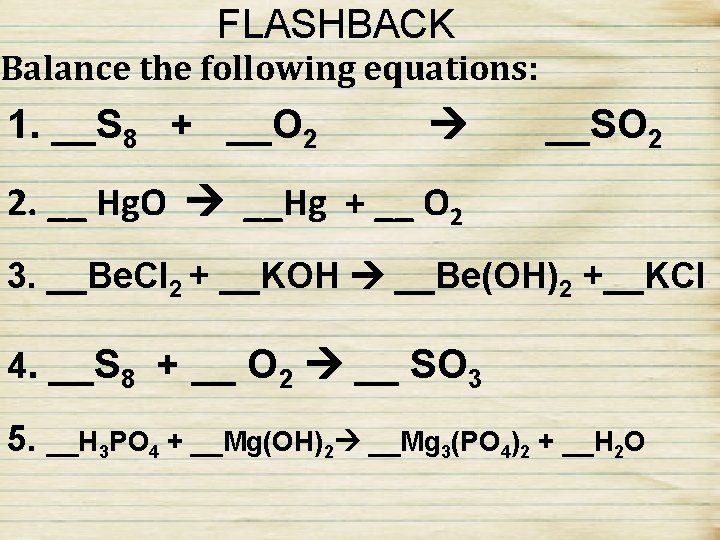
FLASHBACK Balance the following equations: 1. __S 8 + __O 2 __SO 2 2. __ Hg. O __Hg + __ O 2 3. __Be. Cl 2 + __KOH __Be(OH)2 +__KCl 4. __S 8 + __ O 2 __ SO 3 5. __H 3 PO 4 + __Mg(OH)2 __Mg 3(PO 4)2 + __H 2 O

Reaction Rates! Reactions occur when particles of reactants collide with energy

FACTORS AFFECTING REACTION RATES 1. Temperature: higher temperature, reaction rate increases 1. Particles moving faster, more collision between particles 2. Concentration: when reactants are more concentrated, rate of reaction increases 1. More particles mean more collisions

FACTORS AFFECTING REACTION RATES 3. Surface Area: more surface area, reaction rate increases 4. Catalyst: presence of catalyst speeds up reaction without being permanently changed [Inhibitor: slows down a reaction]
![THURSDAY 1022 BELLRINGER EOC WORKBOOK Pg 45 2 6 Pg 46 THURSDAY 10/22 - BELLRINGER EOC WORKBOOK Pg. 45 [# 2 -6] Pg. 46 [#](https://slidetodoc.com/presentation_image_h2/aa3a37cd9d0f0cdee545a2d50f42e33a/image-47.jpg)
THURSDAY 10/22 - BELLRINGER EOC WORKBOOK Pg. 45 [# 2 -6] Pg. 46 [# 1 -6]

Salt: Up close and personal Make some observations of salt under a microscope!! • http: //www. sciencenetlinks. com/lessons. php? Bench mark. ID=4&Doc. ID=173 • http: //www. mos. org/sln/sem. html
