Digestion of dietary proteins Digestion of dietary proteins







- Slides: 13

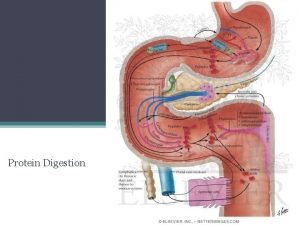
Digestion of dietary proteins

Digestion of dietary proteins l l Most of the nitrogen in the diet is consumed in the form of protein. Protein are generally too large to be absorbed by the intestine. They must be hydrolyzed to yield their constituent amino acid, which can be absorbed.

Proteolytic enzymes responsible for Degrading proteins are produced by three different organs: l l l The stomach The pancreas and the small intestine

Digestion of proteins by gastric secretion The digestion of proteins begins in the stomach, which secretes gastric juiceaunique solution containing: 1 - Hydrochloric acid 2 - Proenzyme, Pepsinogen. l

Hydrochloric acid l l Stomach acid is too dilute (PH 2 to 3) to hydrolyze proteins. The acid function instead to kill bacteria and to denature proteins.

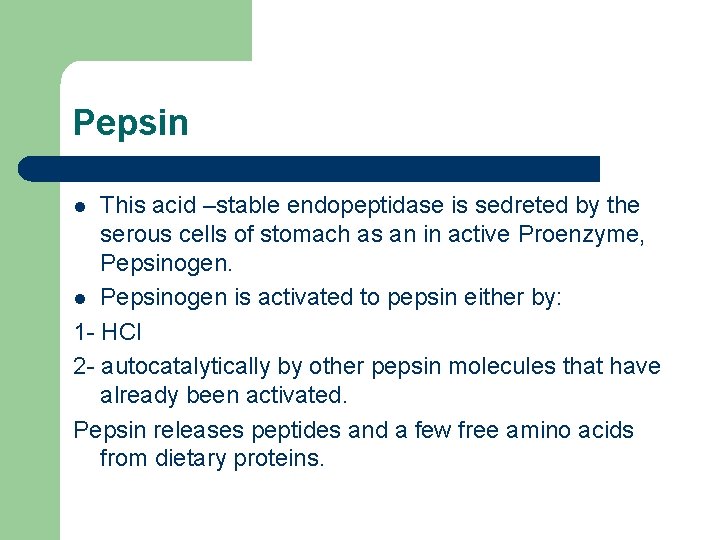
Pepsin This acid –stable endopeptidase is sedreted by the serous cells of stomach as an in active Proenzyme, Pepsinogen. l Pepsinogen is activated to pepsin either by: 1 - HCl 2 - autocatalytically by other pepsin molecules that have already been activated. Pepsin releases peptides and a few free amino acids from dietary proteins. l

Digestion of proteins by pancreatic enzymes l l The release and activation of the pancreatic zymogens is mediated by secretion of Cholecystokinin and Secretin, two polypeptide hormones. pancreatic enzymes has a different specificity for the amino acid R-groups adjacent to the susceptible peptide bond.

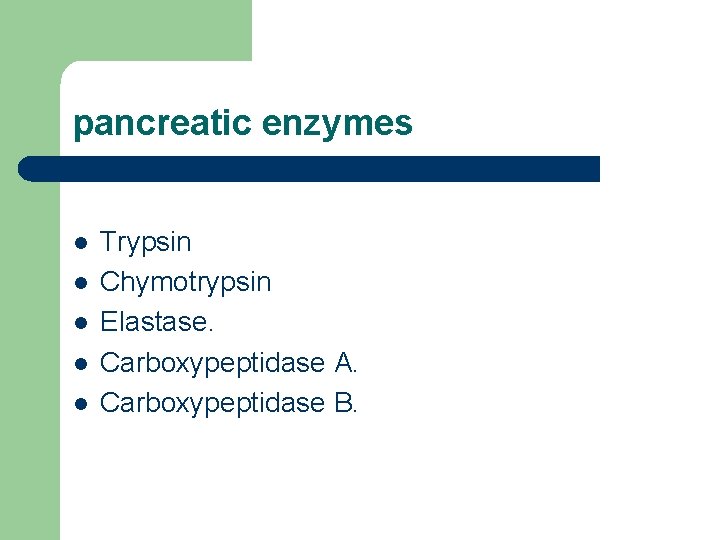
pancreatic enzymes l l l Trypsin Chymotrypsin Elastase. Carboxypeptidase A. Carboxypeptidase B.

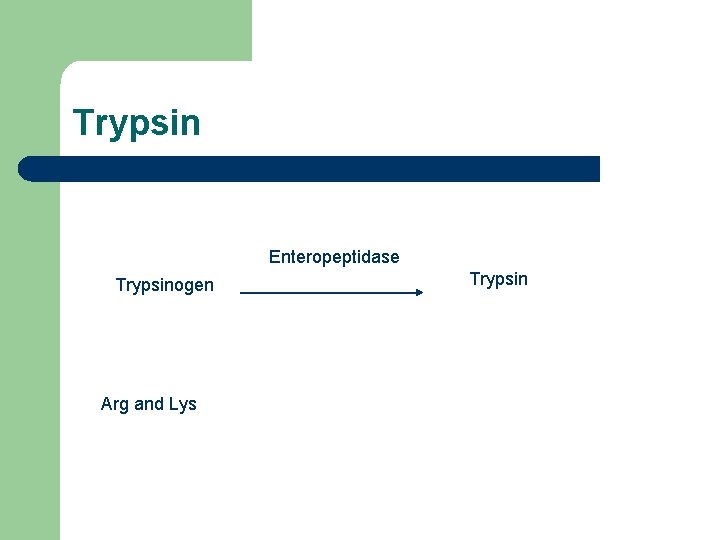
Trypsin Enteropeptidase Trypsinogen Arg and Lys Trypsin

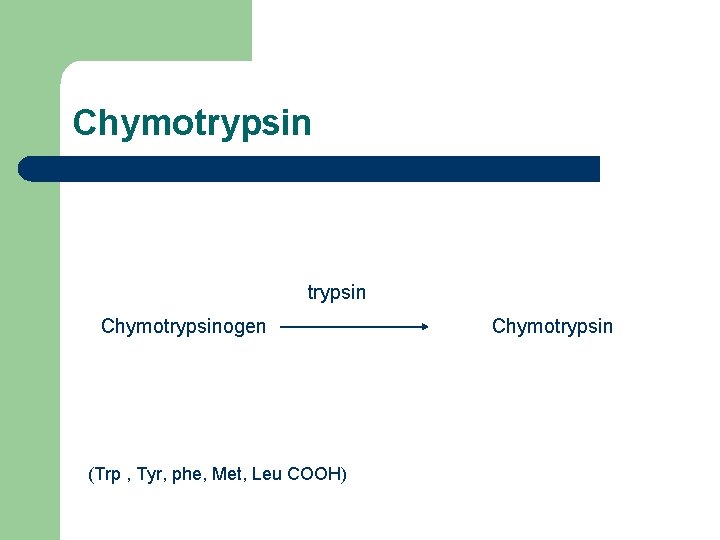
Chymotrypsinogen (Trp , Tyr, phe, Met, Leu COOH) Chymotrypsin

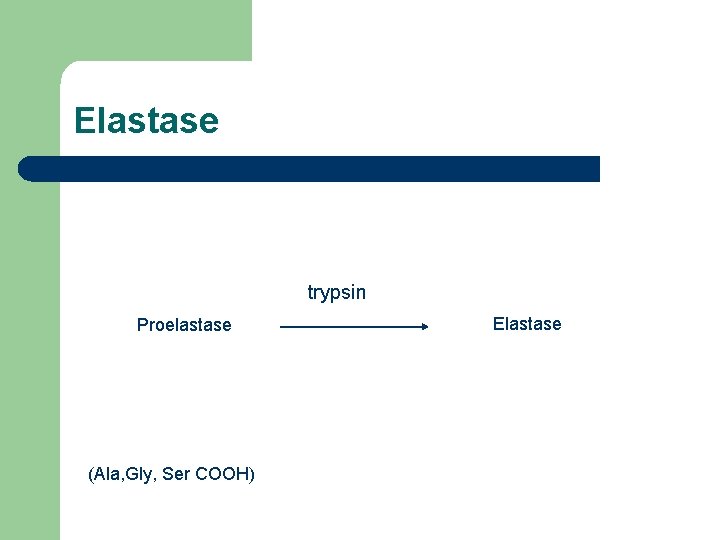
Elastase trypsin Proelastase (Ala, Gly, Ser COOH) Elastase

Carboxypeptidase A Carboxypeptidase B Pro. Carboxypeptidase A. Pro. Carboxypeptidase B. A= (Ala, lle, Leu, Val, NH 2) trypsin B= (Arg, Lys, NH 2) Carboxypeptidase A. Carboxypeptidase B.

Digestion of oligopeptides by enzymes of the small intestine l The luminnal surface of the intestine contains aminopeptidase – an exopeptidase that repeatedly cleaves the N-terminal residue from oligopeptides to produce free amino acids and smaller peptides.
 Venn diagram mechanical and chemical digestion
Venn diagram mechanical and chemical digestion Iron deficiency anemia
Iron deficiency anemia Eating habits questionnaire for students
Eating habits questionnaire for students Sepsis dietary management
Sepsis dietary management Dietary supplement meaning
Dietary supplement meaning Lymfangiectasie
Lymfangiectasie Nutraceutical formulation
Nutraceutical formulation Dietary manipulation in sport definition
Dietary manipulation in sport definition Vitamin k dietary sources
Vitamin k dietary sources Scottish dietary goals
Scottish dietary goals The food exchange system
The food exchange system Hong kong dietary guidelines
Hong kong dietary guidelines Dietary acculturation
Dietary acculturation Buddhist dietary restrictions
Buddhist dietary restrictions