Diffusion Osmosis Define Diffusion The movement of molecules










- Slides: 15

Diffusion & Osmosis

Define Diffusion The movement of molecules from a area in which they are highly concentrated to a area in which they are less concentrated.

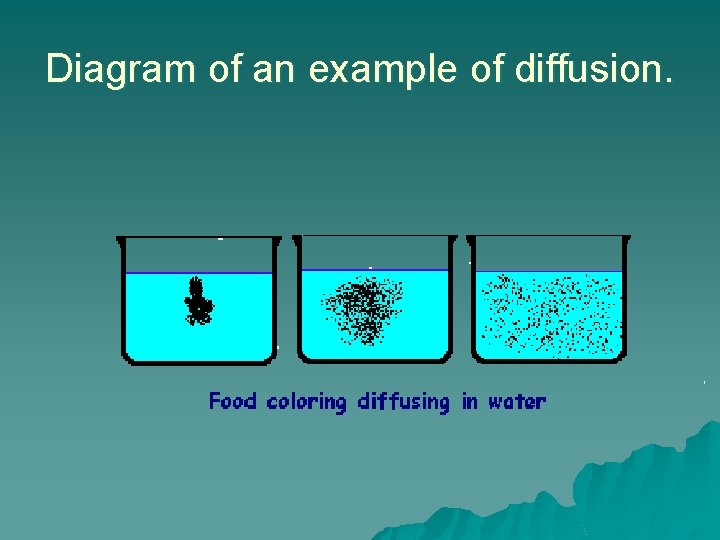
Diagram of an example of diffusion.

See an animation of diffusion here: http: //highered. mcgrawhill. com/sites/0072495855 /student_view 0/chapter 2/ animation__how_diffusion _works. html

Define osmosis u The diffusion of water across a selectively permeable membrane.

Define osmosis Water moves from a high concentration of water (less salt or sugar dissolved in it) to a low concentration of water (more salt or sugar dissolved in it). u This means that water would cross a selectively permeable membrane from a dilute solution (less dissolved in it) to a concentrated solution (more dissolved in it). u http: //www. usd. edu/~bgoodman/Osmos. htm

Osmosis http: //www. usd. edu/~bgoodman/Osmos. htm

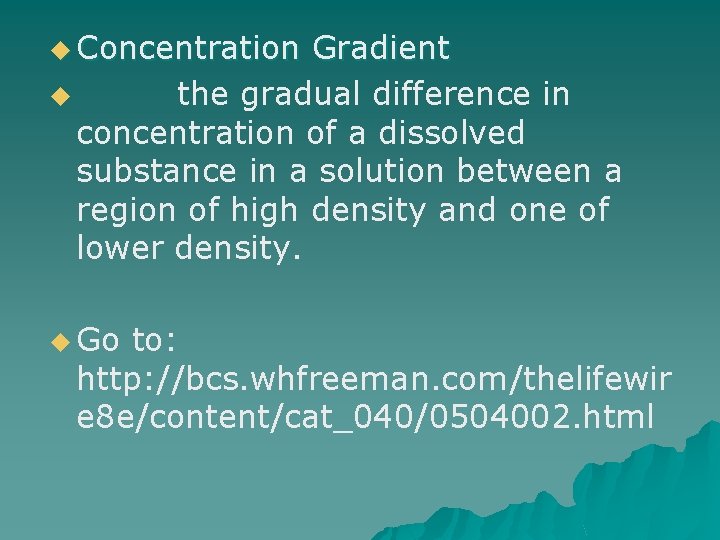
u Concentration Gradient u the gradual difference in concentration of a dissolved substance in a solution between a region of high density and one of lower density. u Go to: http: //bcs. whfreeman. com/thelifewir e 8 e/content/cat_040/0504002. html

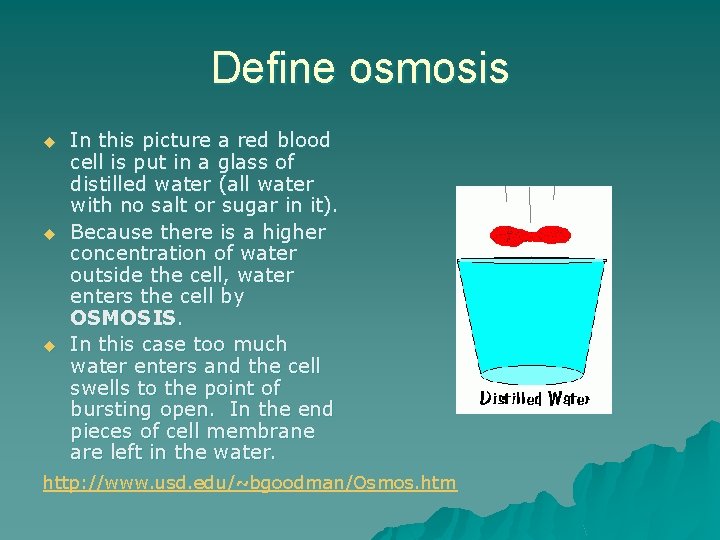
Define osmosis u u u In this picture a red blood cell is put in a glass of distilled water (all water with no salt or sugar in it). Because there is a higher concentration of water outside the cell, water enters the cell by OSMOSIS. In this case too much water enters and the cell swells to the point of bursting open. In the end pieces of cell membrane are left in the water. http: //www. usd. edu/~bgoodman/Osmos. htm

See an animation of osmosis here: http: //highered. mcgrawhill. com/sites/0072495855 /student_view 0/chapter 2/ animation__how_diffusion _works. html

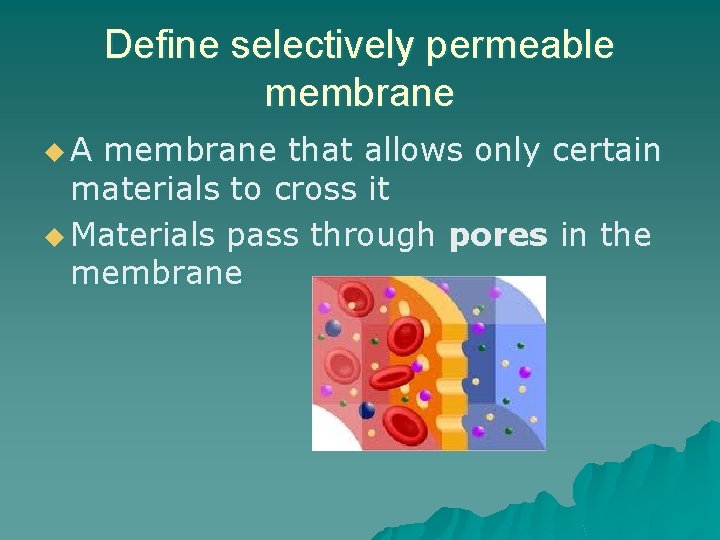
Define selectively permeable membrane u. A membrane that allows only certain materials to cross it u Materials pass through pores in the membrane

Why are osmosis & diffusion important?

Why are osmosis & diffusion important? All living things have certain requirements they must satisfy in order to remain alive – maintain homeostasis u These include exchanging gases (usually CO 2 and O 2), taking in water, minerals, and food, and eliminating wastes. u These tasks happen at the cellular level. u Molecules move through the cell membrane by diffusion u

Why are osmosis & diffusion important? u All living things have certain requirements they must satisfy in order to remain alive. u These a) b) c) include: exchanging gases (usually CO 2 and O 2) taking in water, minerals, and food, and eliminating wastes.

Why are osmosis & diffusion important? u This membrane is a complex structure that is responsible for u A) separating the contents of the cell from its surroundings, u B)for controlling the movement of materials into and out of the cell, u C)for interacting with the environment surrounding the cell.