Diffusion Osmosis and the Cell Membrane The process
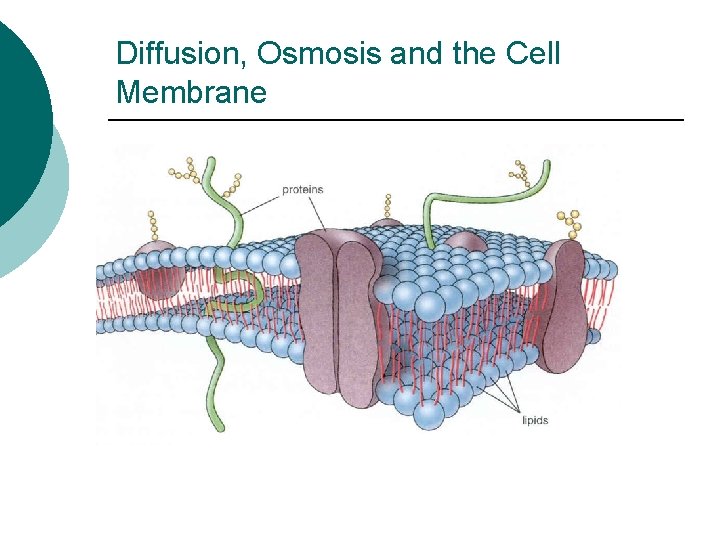
Diffusion, Osmosis and the Cell Membrane
The process of diffusion • Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. • Diffusion results because of the random movement of particles called Brownian motion. • Three key factors affect the rate of diffusion: concentration, temperature, and pressure
Concentration Gradients • The difference in concentration of a substance across a space is called a concentration gradient. • Substances diffuse from an area of higher concentration to an area of lower concentration, moving with the gradient. • Dynamic equilibrium occurs when there is no longer a concentration gradient.
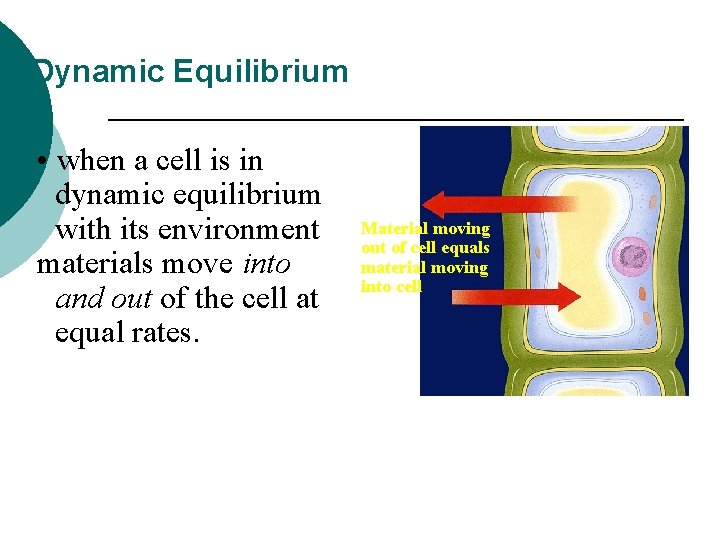
Dynamic Equilibrium • when a cell is in dynamic equilibrium with its environment materials move into and out of the cell at equal rates. Material moving out of cell equals material moving into cell
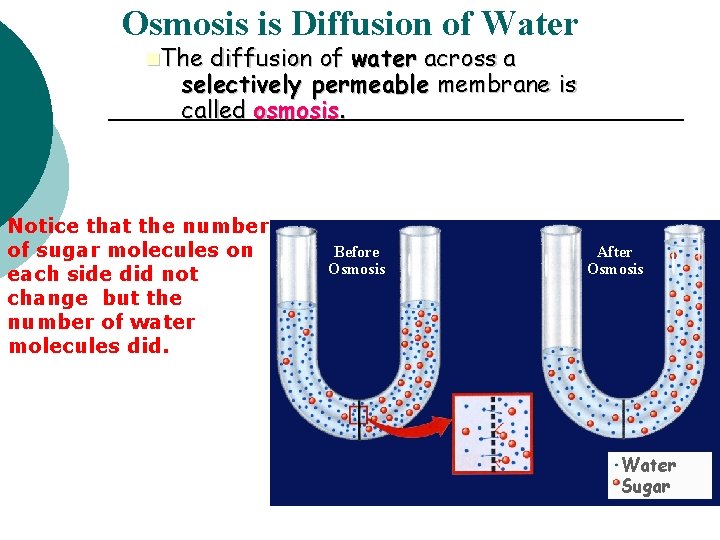
Osmosis is Diffusion of Water n. The diffusion of water across a selectively permeable membrane is called osmosis. Notice that the number of sugar molecules on each side did not change but the number of water molecules did. Before Osmosis After Osmosis Water Sugar
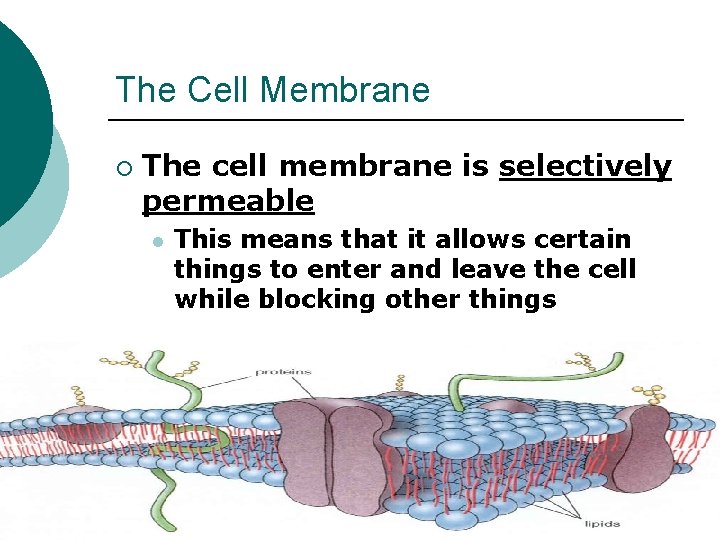
The Cell Membrane ¡ The cell membrane is selectively permeable l This means that it allows certain things to enter and leave the cell while blocking other things
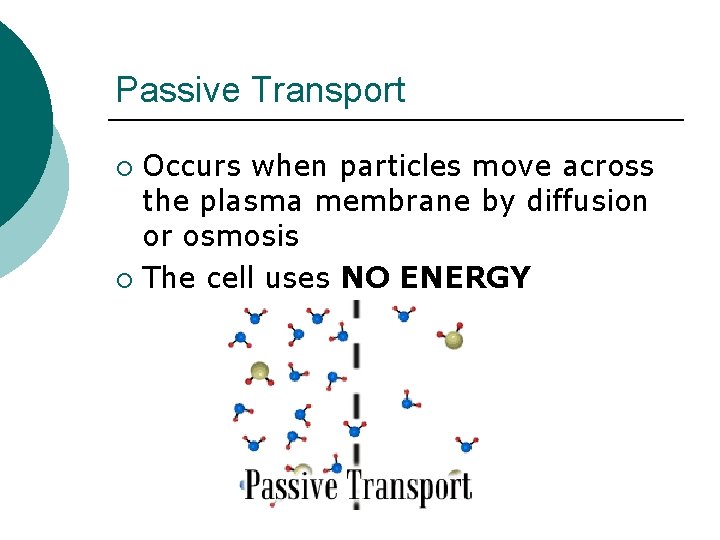
Passive Transport Occurs when particles move across the plasma membrane by diffusion or osmosis ¡ The cell uses NO ENERGY ¡
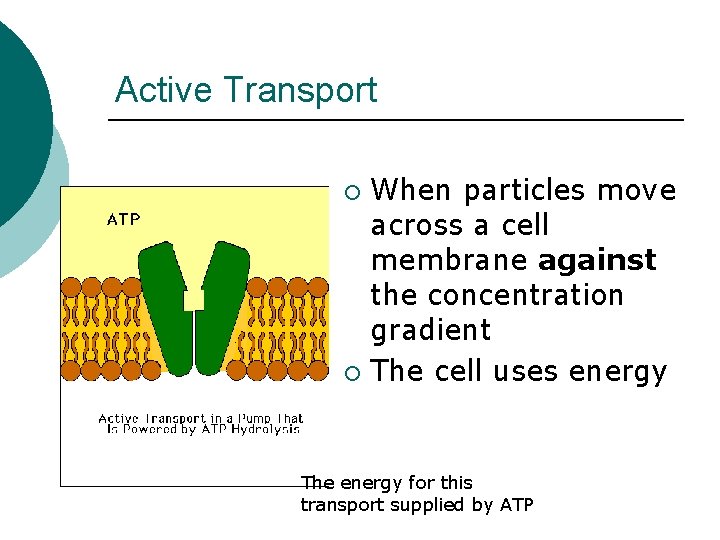
Active Transport When particles move across a cell membrane against the concentration gradient ¡ The cell uses energy ¡ The energy for this transport supplied by ATP

Osmosis in living cells H 2 O Water Molecule Dissolved Molecule Most cells whether in multicellular or unicellular organisms, are subject to osmosis because they are surrounded by water solutions.
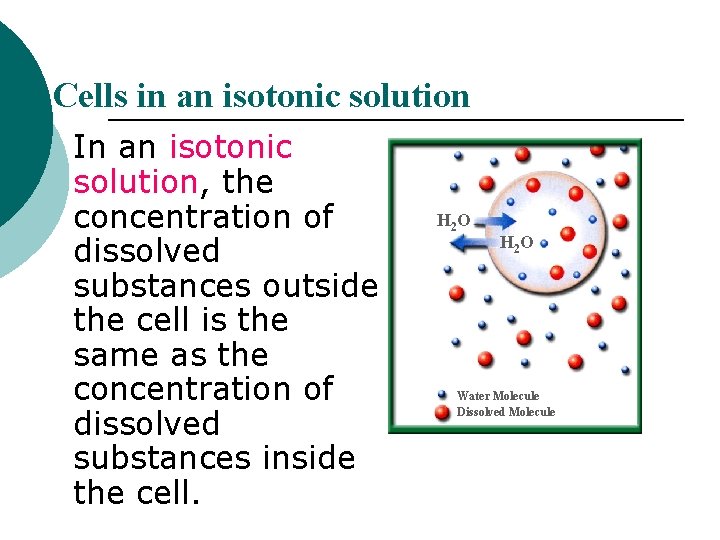
Cells in an isotonic solution In an isotonic solution, the concentration of dissolved substances outside the cell is the same as the concentration of dissolved substances inside the cell. H 2 O Water Molecule Dissolved Molecule
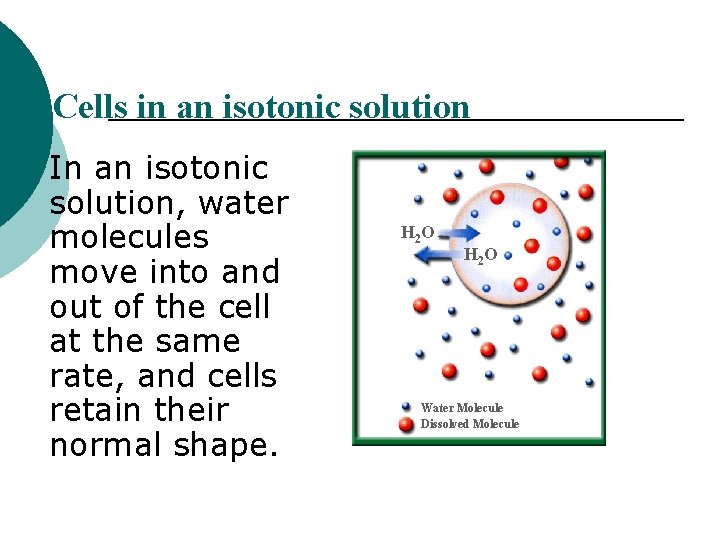
Cells in an isotonic solution In an isotonic solution, water molecules move into and out of the cell at the same rate, and cells retain their normal shape. H 2 O Water Molecule Dissolved Molecule
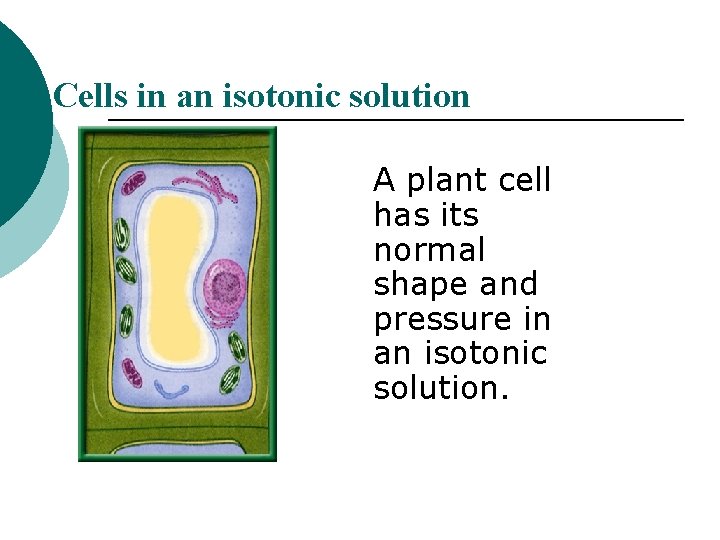
Cells in an isotonic solution A plant cell has its normal shape and pressure in an isotonic solution.
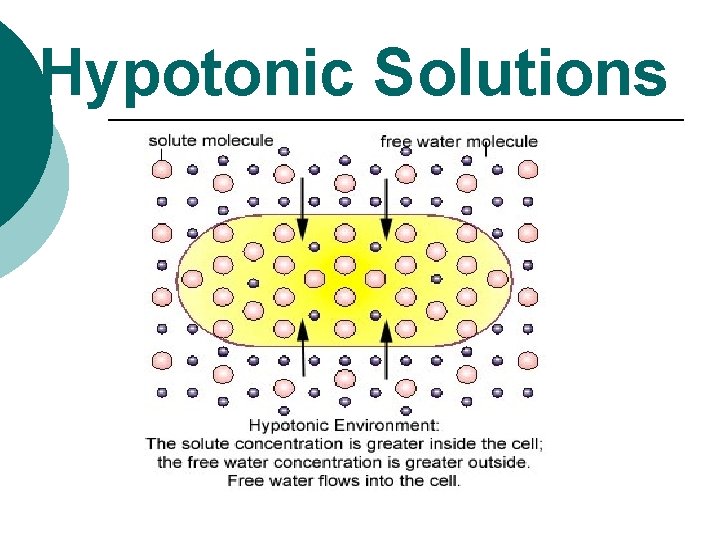
Hypotonic Solutions
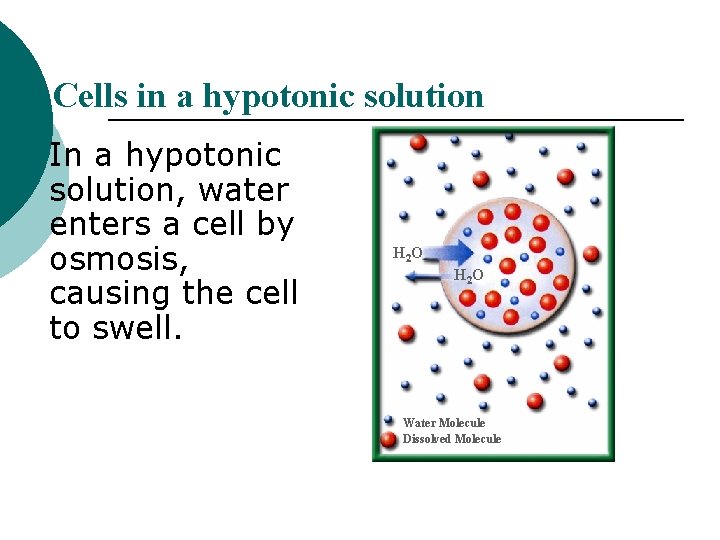
Cells in a hypotonic solution In a hypotonic solution, water enters a cell by osmosis, causing the cell to swell. H 2 O Water Molecule Dissolved Molecule
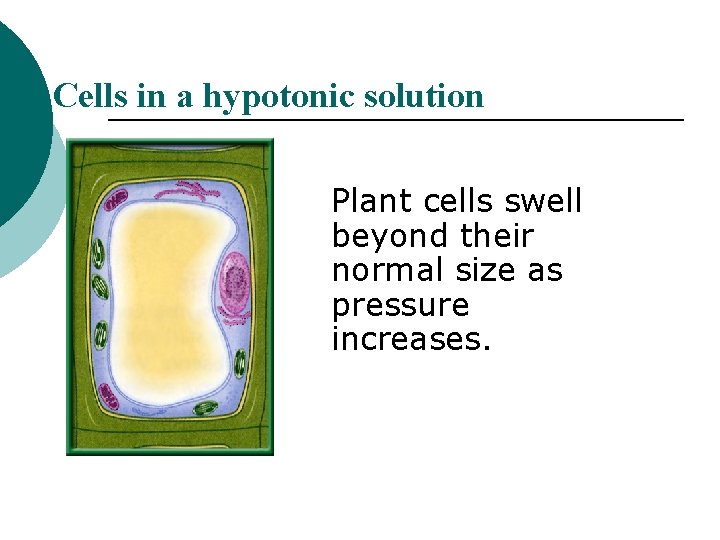
Cells in a hypotonic solution Plant cells swell beyond their normal size as pressure increases.
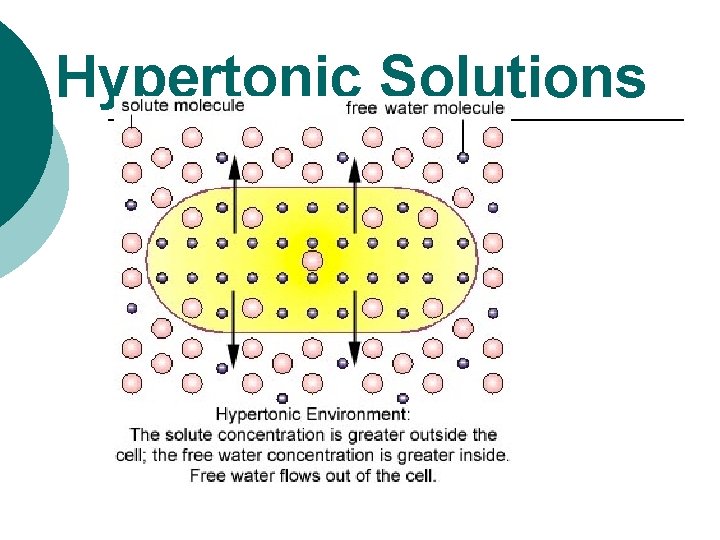
Hypertonic Solutions
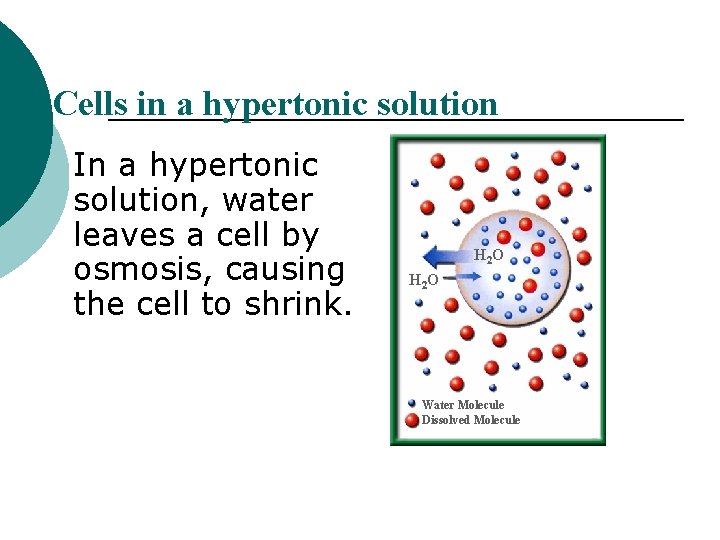
Cells in a hypertonic solution In a hypertonic solution, water leaves a cell by osmosis, causing the cell to shrink. H 2 O Water Molecule Dissolved Molecule
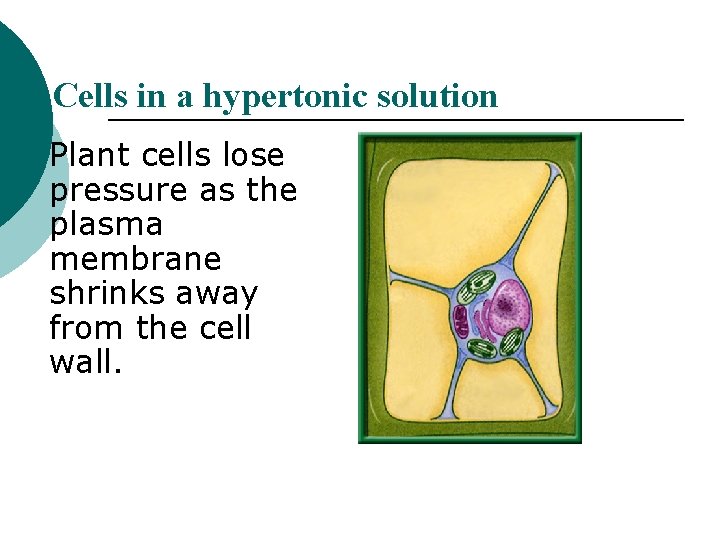
Cells in a hypertonic solution Plant cells lose pressure as the plasma membrane shrinks away from the cell wall.
- Slides: 18