Diffusion in multiphase systems Authors Honorata Kazimierczak Jagoda





![Fick’s first law J = flux [atoms m-2 s-1] = concentration gradient [atoms m-4] Fick’s first law J = flux [atoms m-2 s-1] = concentration gradient [atoms m-4]](https://slidetodoc.com/presentation_image/42ac9c3a53fb7302f6d84defceaebff3/image-16.jpg)













- Slides: 64

Diffusion in multiphase systems Authors: Honorata Kazimierczak Jagoda Poplewska Piotr Bobrowski Marta Gajewska Grażyna Kulesza Katarzyna Stan Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Content 1. 2. 3. 4. 5. 6. Definition of diffusion, Fick’s laws Diffusion in multiphase binary system The Matano-Boltzmann method Intrinsic diffusion coefficient Radiotracer diffusion coefficient Wagner’s integral interdiffusion coefficient Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Diffusion in multiphase systems 1. Definition of diffusion, Fick’s laws Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Diffusion - mass transport by atomic motion. Conditions necessary for diffusion to occur: - an empty adjacent site - enough energy to break bonds and cause lattice distortions during displacement Types of diffusion in solids • Self-diffusion — movement of atoms through their own lattice • Interdiffusion (a. k. a. impurity/chemical diffusion) — when material diffuses from A to B and from B to A. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

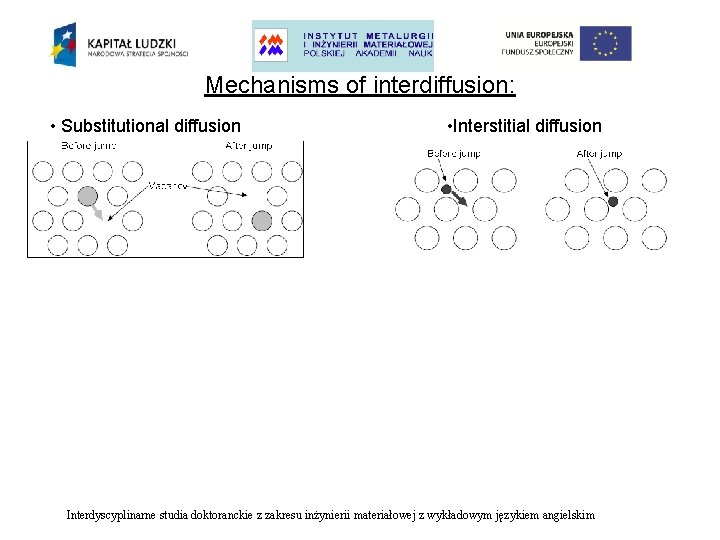
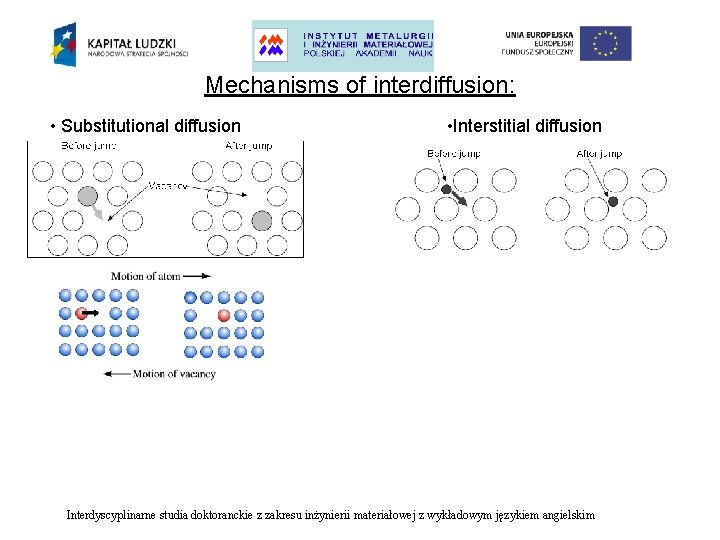
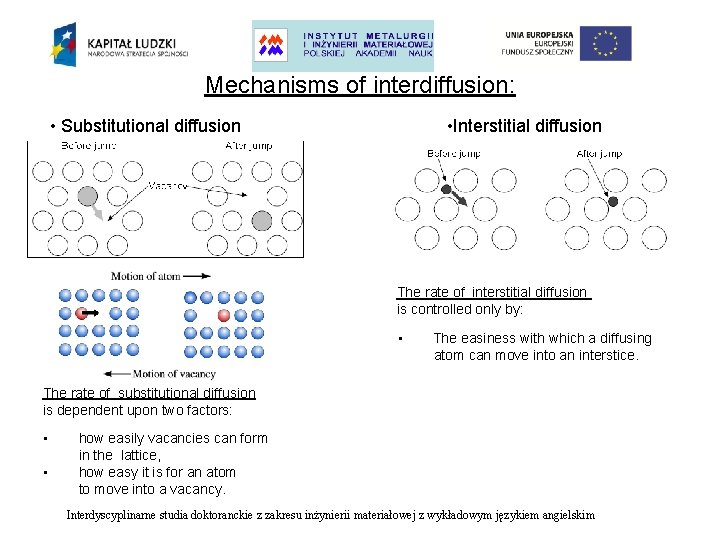
Mechanisms of interdiffusion: • Substitutional diffusion • Interstitial diffusion Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Mechanisms of interdiffusion: • Substitutional diffusion • Interstitial diffusion Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

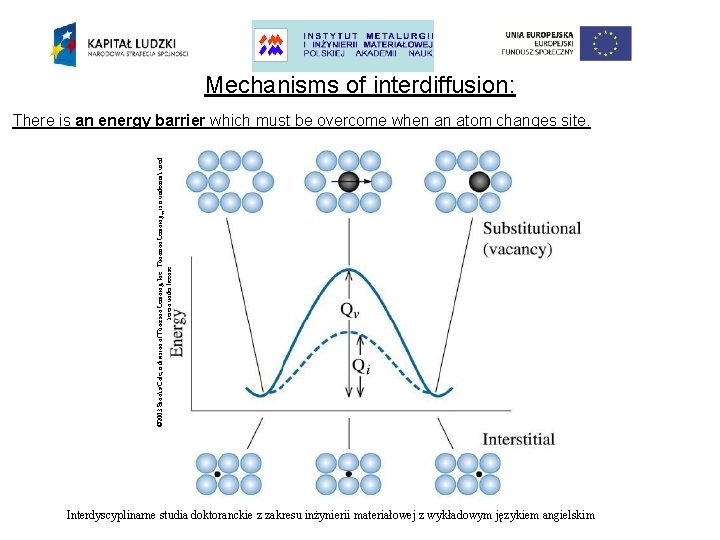
Mechanisms of interdiffusion: • Substitutional diffusion • Interstitial diffusion The rate of interstitial diffusion is controlled only by: • The easiness with which a diffusing atom can move into an interstice. The rate of substitutional diffusion is dependent upon two factors: • • how easily vacancies can form in the lattice, how easy it is for an atom to move into a vacancy. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

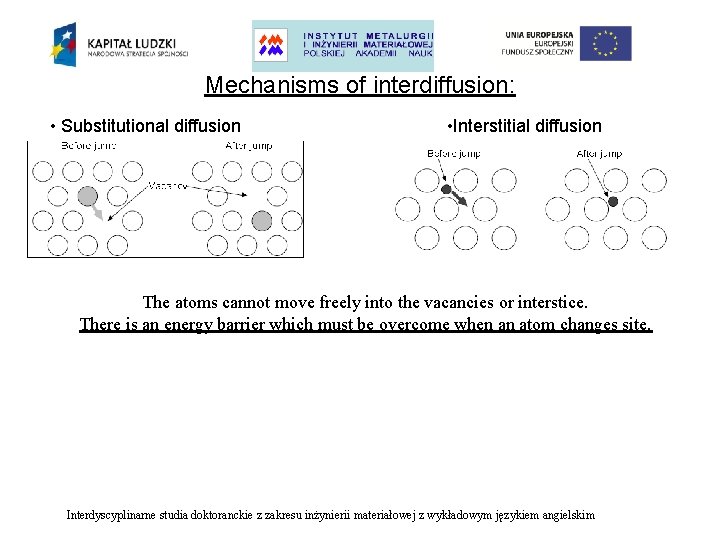
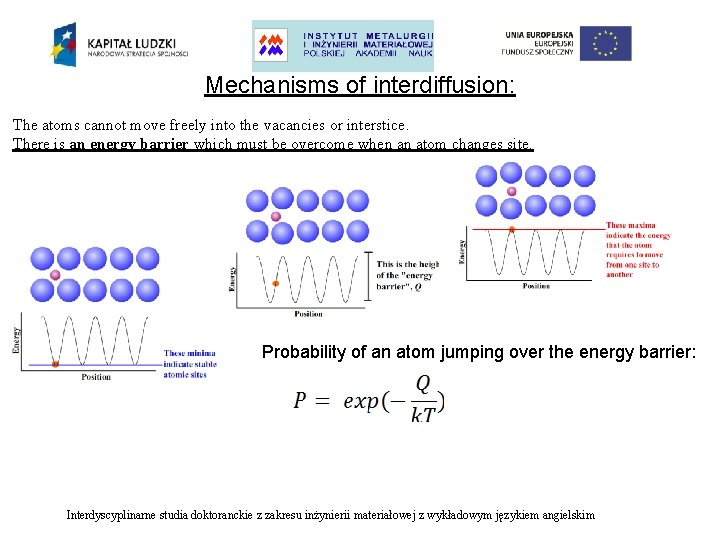
Mechanisms of interdiffusion: • Substitutional diffusion • Interstitial diffusion The atoms cannot move freely into the vacancies or interstice. There is an energy barrier which must be overcome when an atom changes site. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Mechanisms of interdiffusion: The atoms cannot move freely into the vacancies or interstice. There is an energy barrier which must be overcome when an atom changes site. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Mechanisms of interdiffusion: The atoms cannot move freely into the vacancies or interstice. There is an energy barrier which must be overcome when an atom changes site. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

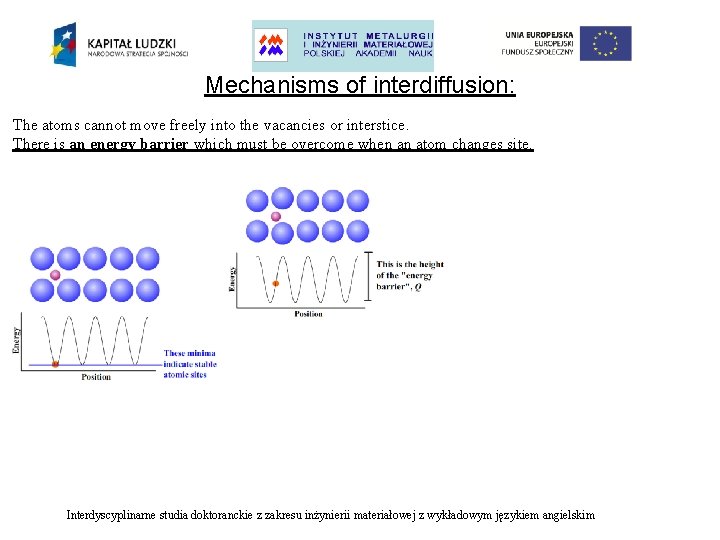
Mechanisms of interdiffusion: The atoms cannot move freely into the vacancies or interstice. There is an energy barrier which must be overcome when an atom changes site. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

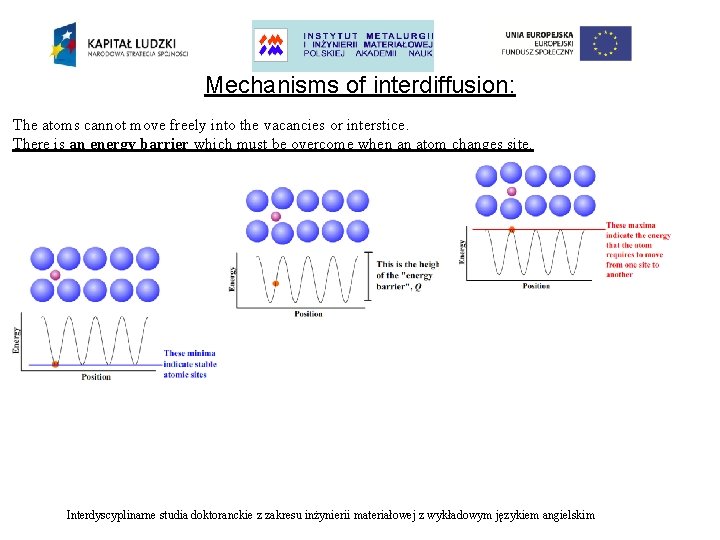
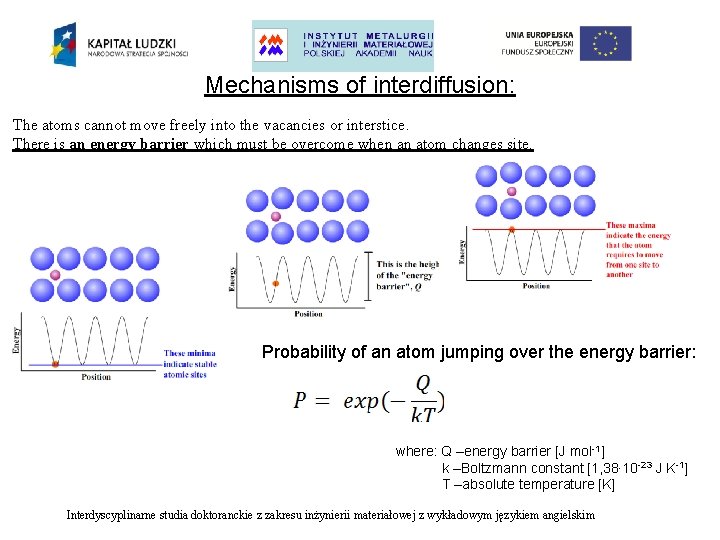
Mechanisms of interdiffusion: The atoms cannot move freely into the vacancies or interstice. There is an energy barrier which must be overcome when an atom changes site. Probability of an atom jumping over the energy barrier: Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Mechanisms of interdiffusion: The atoms cannot move freely into the vacancies or interstice. There is an energy barrier which must be overcome when an atom changes site. Probability of an atom jumping over the energy barrier: where: Q –energy barrier [J mol-1] k –Boltzmann constant [1, 38∙ 10 -23 J K-1] T –absolute temperature [K] Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Mechanisms of interdiffusion: There is an energy barrier which must be overcome when an atom changes site. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

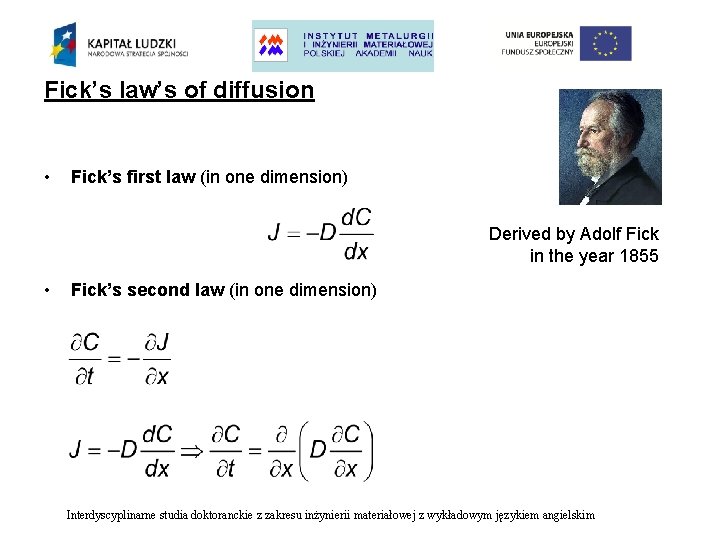
Fick’s law’s of diffusion • Fick’s first law (in one dimension) Derived by Adolf Fick in the year 1855 • Fick’s second law (in one dimension) Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim
![Ficks first law J flux atoms m2 s1 concentration gradient atoms m4 Fick’s first law J = flux [atoms m-2 s-1] = concentration gradient [atoms m-4]](https://slidetodoc.com/presentation_image/42ac9c3a53fb7302f6d84defceaebff3/image-16.jpg)
Fick’s first law J = flux [atoms m-2 s-1] = concentration gradient [atoms m-4] D = diffusion coefficient [m 2 s-1] Fick’s 1 st law can only be used to solve steady-state diffusion problems. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

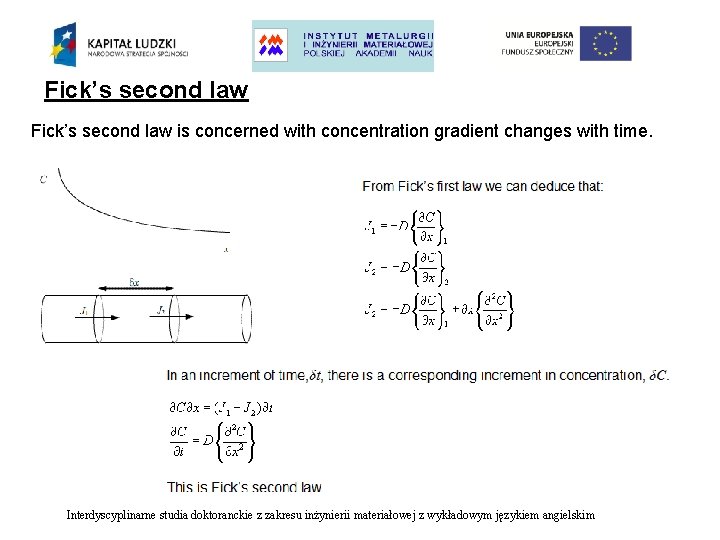
Fick’s second law is concerned with concentration gradient changes with time. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

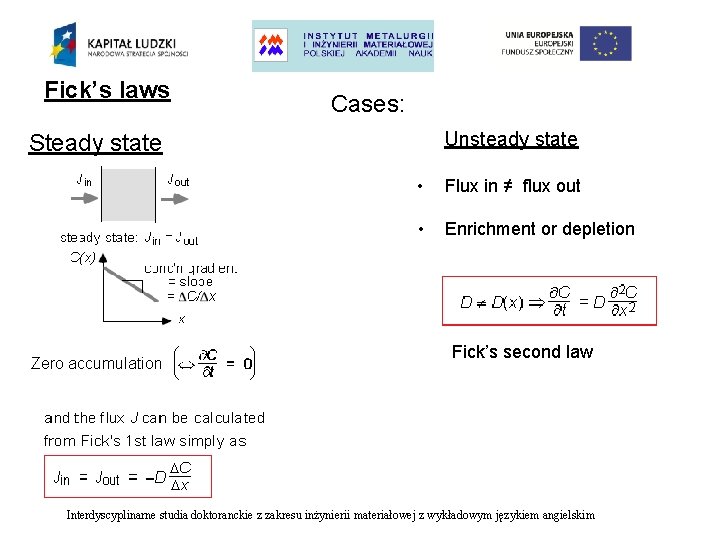
Fick’s laws Cases: Unsteady state Steady state Zero accumulation • Flux in ≠ flux out • Enrichment or depletion Fick’s second law Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Diffusion in multiphase systems 2. Diffusion in Multiphase Binary System Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

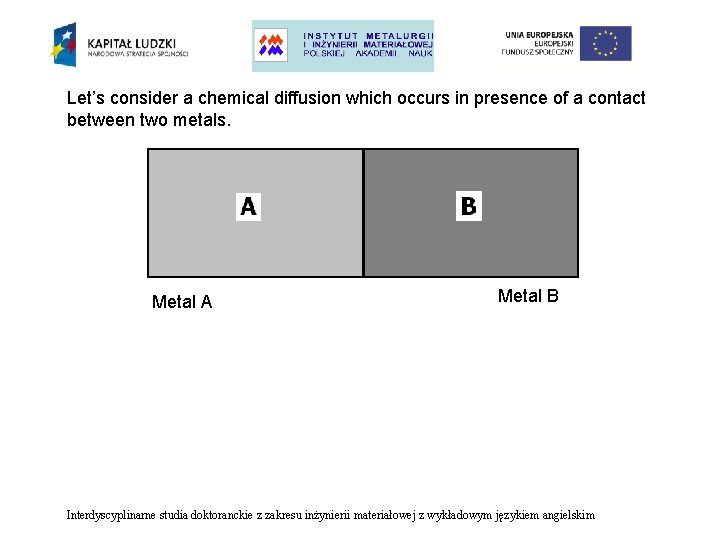
Let’s consider a chemical diffusion which occurs in presence of a contact between two metals. Metal A Metal B Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

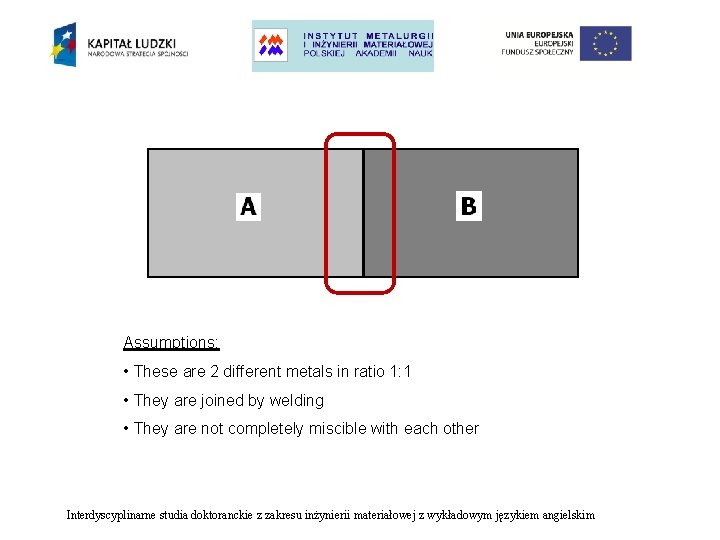
Assumptions: • These are 2 different metals in ratio 1: 1 • They are joined by welding • They are not completely miscible with each other Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

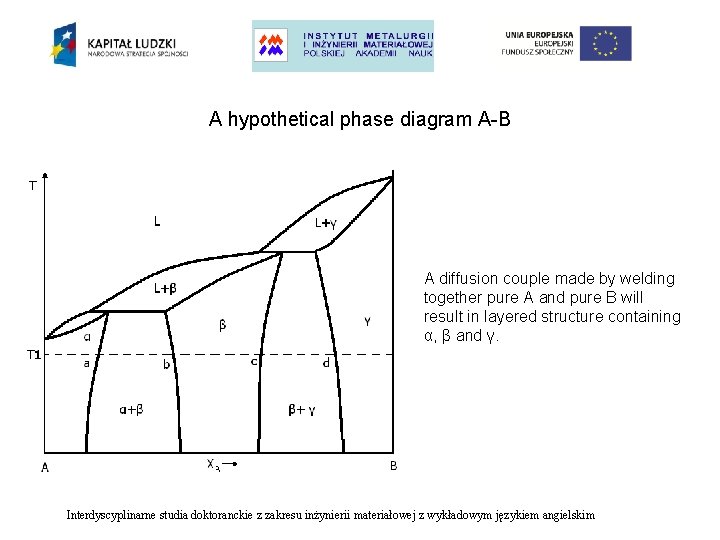
A hypothetical phase diagram A-B A diffusion couple made by welding together pure A and pure B will result in layered structure containing α, β and γ. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

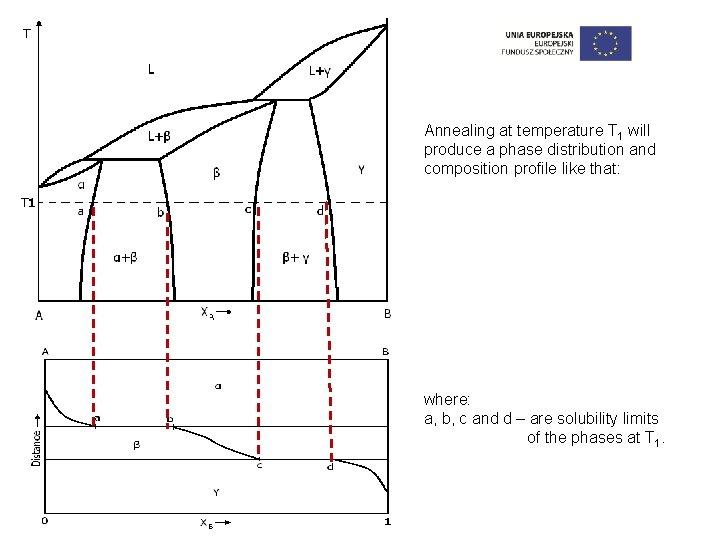
Annealing at temperature T 1 will produce a phase distribution and composition profile like that: where: a, b, c and d – are solubility limits of the phases at T 1.

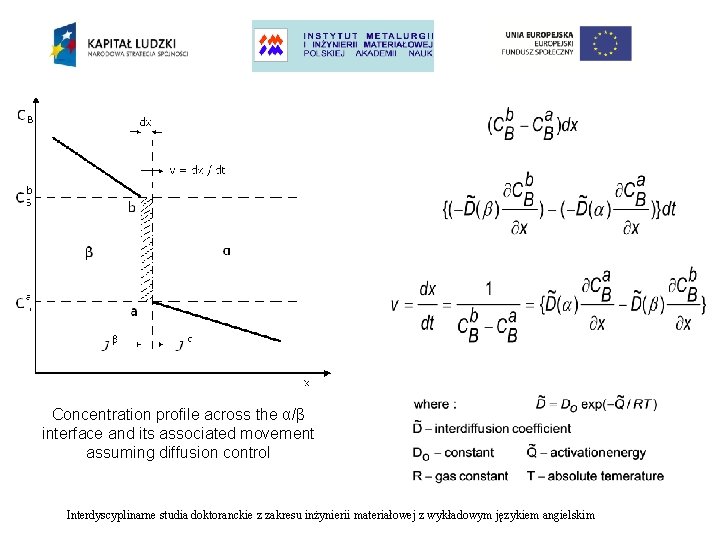
Concentration profile across the α/β interface and its associated movement assuming diffusion control Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

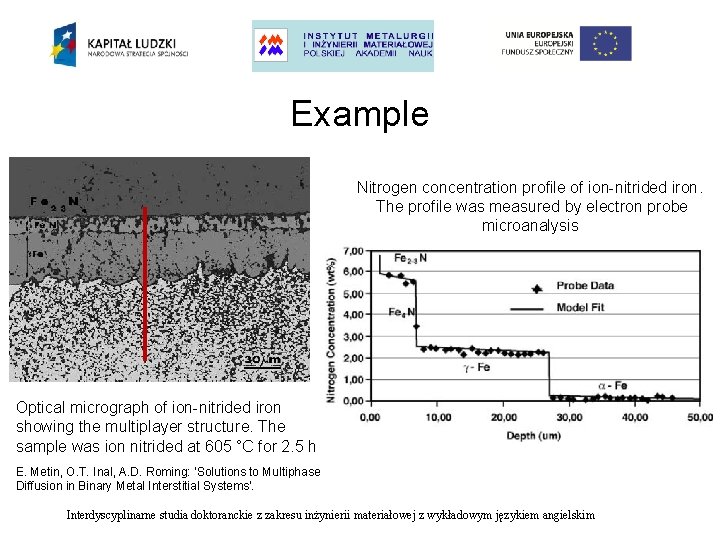
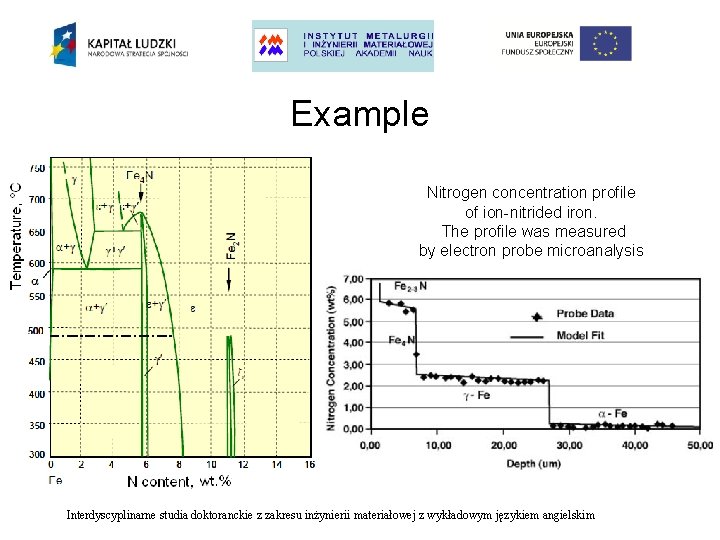
Example Nitrogen concentration profile of ion-nitrided iron. The profile was measured by electron probe microanalysis Optical micrograph of ion-nitrided iron showing the multiplayer structure. The sample was ion nitrided at 605 °C for 2. 5 h E. Metin, O. T. Inal, A. D. Roming: ‘Solutions to Multiphase Diffusion in Binary Metal Interstitial Systems’. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Example Nitrogen concentration profile of ion-nitrided iron. The profile was measured by electron probe microanalysis Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Diffusion in multiphase systems 3. The Matano-Boltzmann method Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

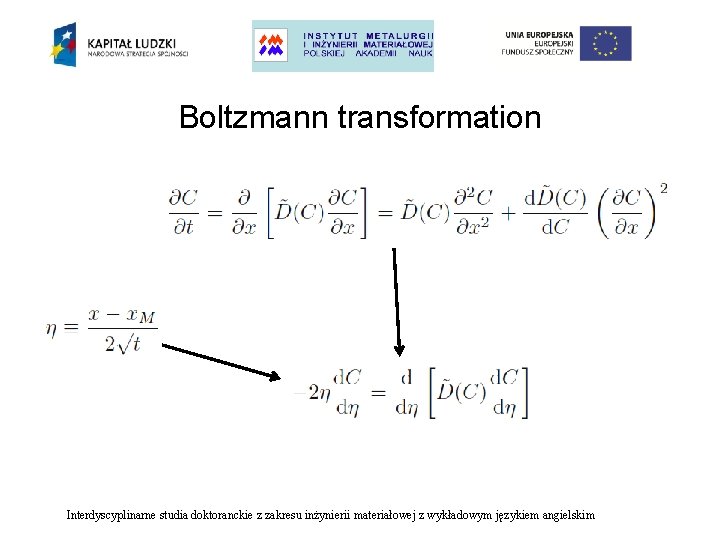
Boltzmann transformation Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

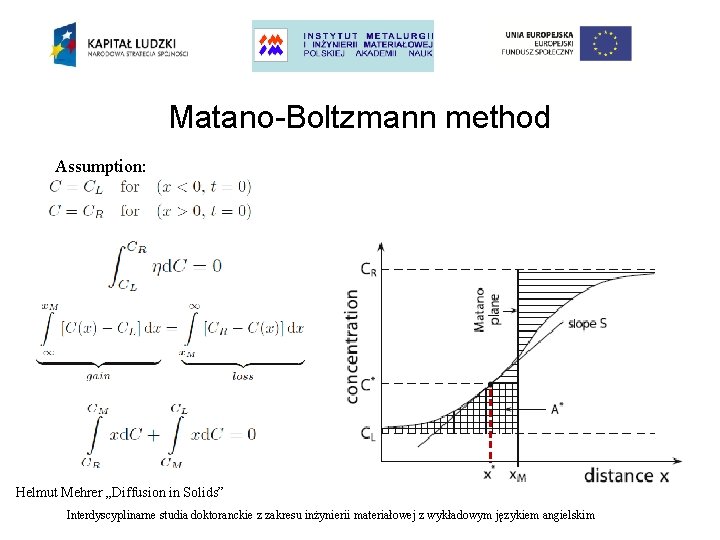
Matano-Boltzmann method Assumption: Helmut Mehrer „Diffusion in Solids” Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

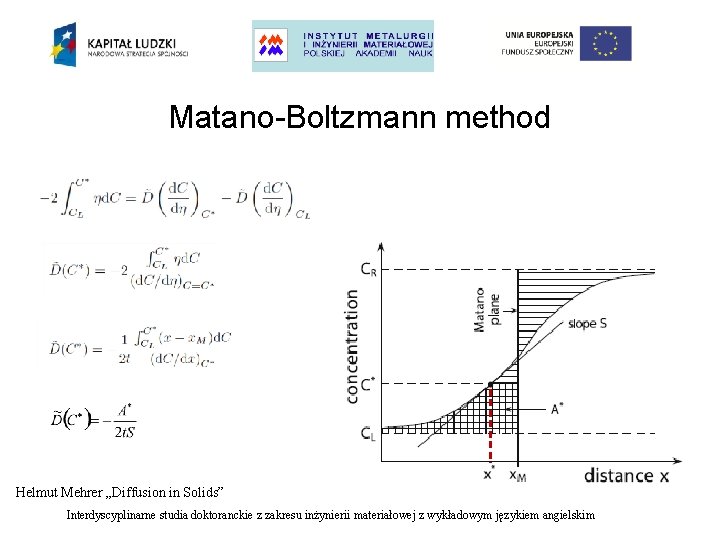
Matano-Boltzmann method Helmut Mehrer „Diffusion in Solids” Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

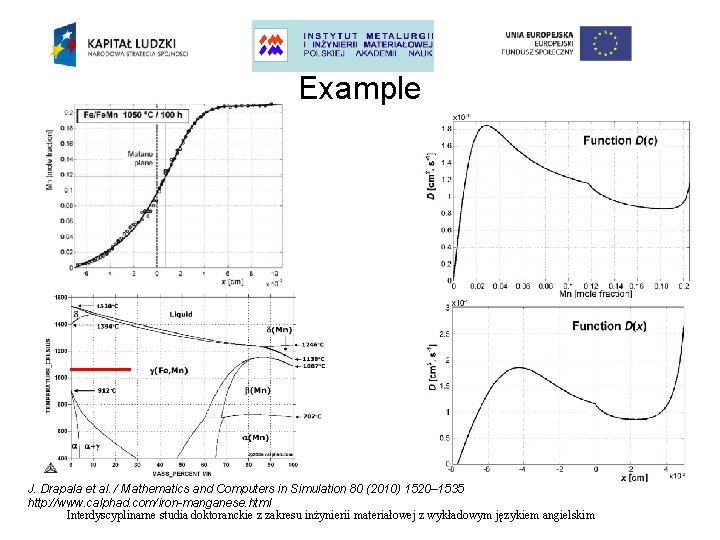
Example J. Drapala et al. / Mathematics and Computers in Simulation 80 (2010) 1520– 1535 http: //www. calphad. com/iron-manganese. html Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Sequent steps in Matano-Boltzmann method: - determine the position of the Matano plane - choose C* and calculate the integral - calculate the concentration gradient - determine diffusion coefficient D Important details: - the method assumes infinite system - at small concentrations the integral and derivative are very small and uncertinities become high - Matano plane is not the same as Kirkendall plane - the method assumes constant volume Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Diffusion in multiphase systems 4. Intrinsic diffusion coefficient Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

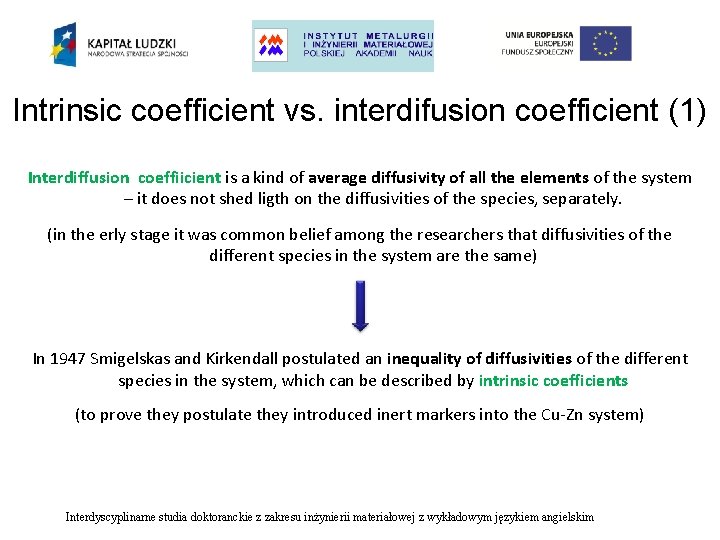
Intrinsic coefficient vs. interdifusion coefficient (1) Interdiffusion coeffiicient is a kind of average diffusivity of all the elements of the system – it does not shed ligth on the diffusivities of the species, separately. (in the erly stage it was common belief among the researchers that diffusivities of the different species in the system are the same) In 1947 Smigelskas and Kirkendall postulated an inequality of diffusivities of the different species in the system, which can be described by intrinsic coefficients (to prove they postulate they introduced inert markers into the Cu-Zn system) Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

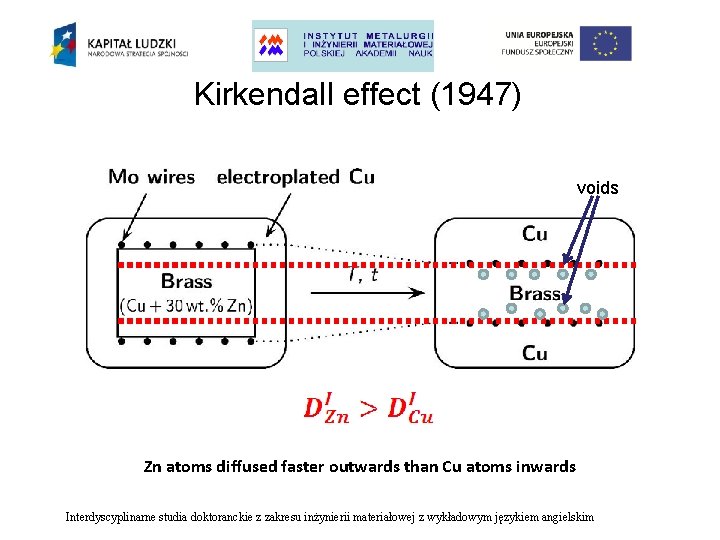
Kirkendall effect (1947) voids Zn atoms diffused faster outwards than Cu atoms inwards Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

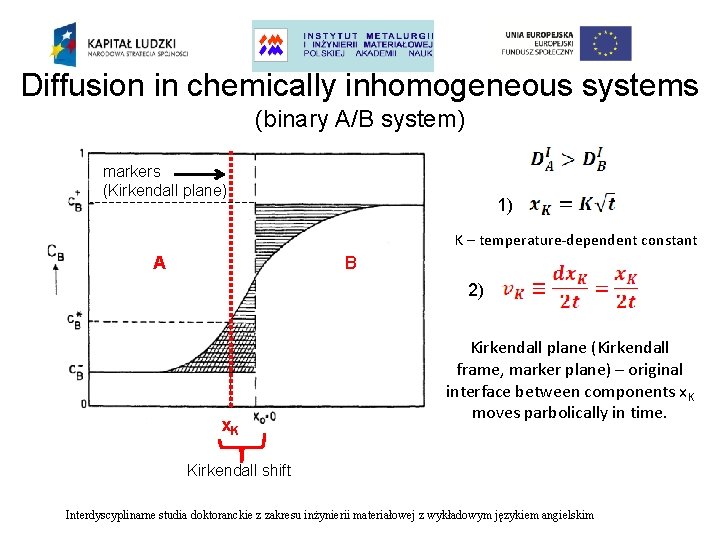
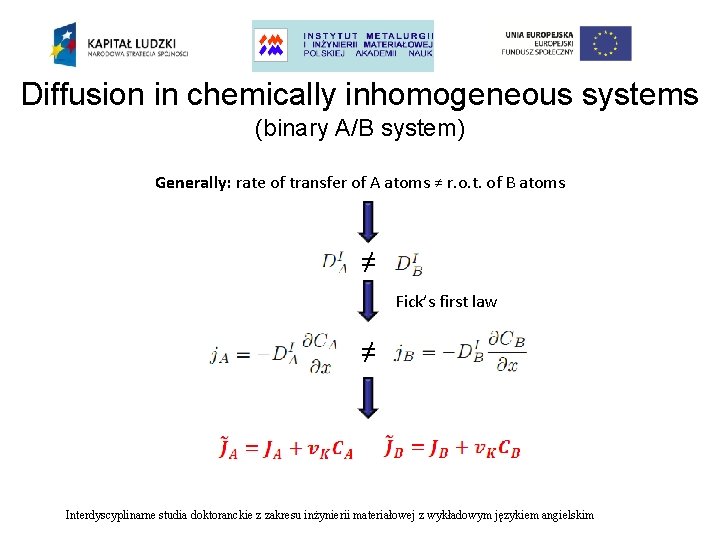
Diffusion in chemically inhomogeneous systems (binary A/B system) markers (Kirkendall plane) 1) K – temperature-dependent constant A B 2) x. K Kirkendall plane (Kirkendall frame, marker plane) – original interface between components x. K moves parbolically in time. Kirkendall shift Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Diffusion in chemically inhomogeneous systems (binary A/B system) Generally: rate of transfer of A atoms ≠ r. o. t. of B atoms ≠ Fick’s first law ≠ Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

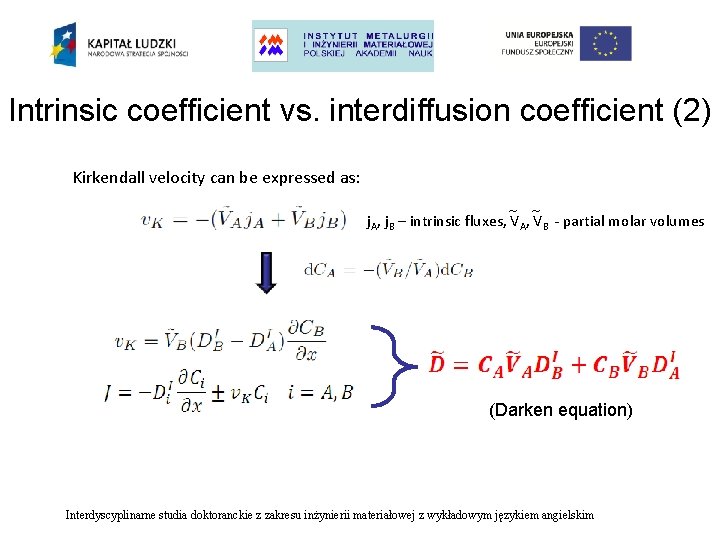
Intrinsic coefficient vs. interdiffusion coefficient (2) Kirkendall velocity can be expressed as: ~ ~ j. A, j. B – intrinsic fluxes, VA, VB - partial molar volumes (Darken equation) Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

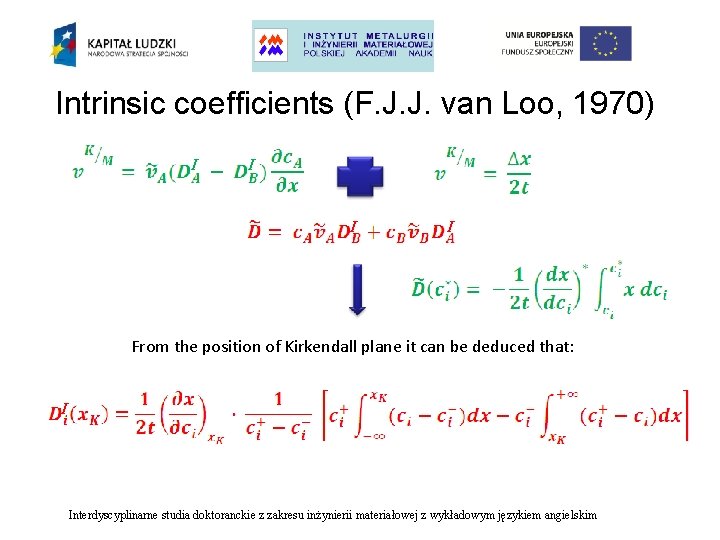
Intrinsic coefficients (F. J. J. van Loo, 1970) I I From the position of Kirkendall plane it can be deduced that: I Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Intrinsic coefficients vs. interdiffusion coefficients IMPORTANT: Interdiffusion coefficients can be measured at any composition in a concentration profile, however, intrinsic diffusivities can only be measured at compositions indicated by inert markers (Kirkendall plane) Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

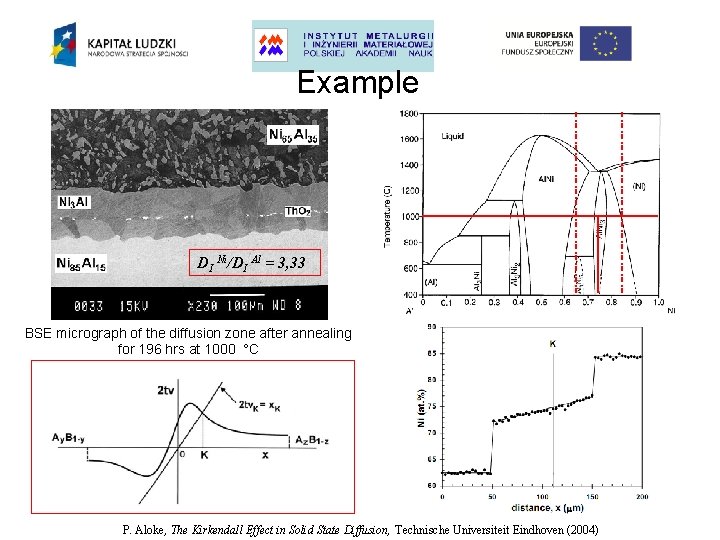
Example DI Ni/DI Al = 3, 33 BSE micrograph of the diffusion zone after annealing for 196 hrs at 1000 °C P. Aloke, The Kirkendall Effect in Solid State Diffusion, Technische Universiteit Eindhoven (2004)

Diffusion in multiphase systems 5. Radiotracer diffusion coefficient Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

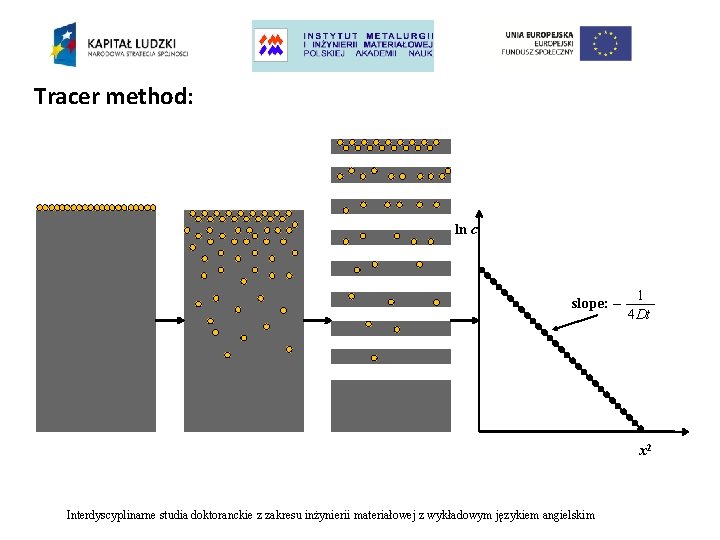
Tracer method: ln c slope: – 1 4 Dt x 2 Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Tracer method: Ø Tracer is deposited onto a polished, flat surface (normally radioactive isotopes are used) Ø Techniques of common deposition: evaporation, dripping of a liquid solution, electrodeposition, ion-implantation as a thin layer below the sample surface in order to overcome disturbing surface oxide hold-up and solubility problem. Ø Sample is encapsulated in quartz ampoule under vacuum or Ar atmosphere Ø Isothermal diffusion anneal is performed in a furnace at the temperature T for some time t (at T=1600 K quartz ampoules cannot be used any more, then more sophisticated techniques are necessary) Ø Serial sectioning of the sample and subsequent determination of the amount of tracer per section. This is the best way to determine the resulting concentration depth profile Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Section techniques: Ø mechanical sectioning: for average diffusion length at least several micrometers : • lathes D > 10 -15 m 2 s-1 • microtome D > 10 -17 m 2 s-1 • grinder sectioning D ≈ 10 -18 m 2 s-1 Ø sputter sectioning: at lower temperature there are very small diffusivities. Sputtering or secondary ion mass spectrometry (SIMS) permit serial sectioning of shallow diffusion zones which corresponds to average of diffusion length between 2 nm and 10 μm, D ≈ 10 -24 m 2 s-1 - D ≈ 10 -16 m 2 s-1 NOTE: For brittle materials (intermetallics, semiconductors, glasses) only grinding method is applicable. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

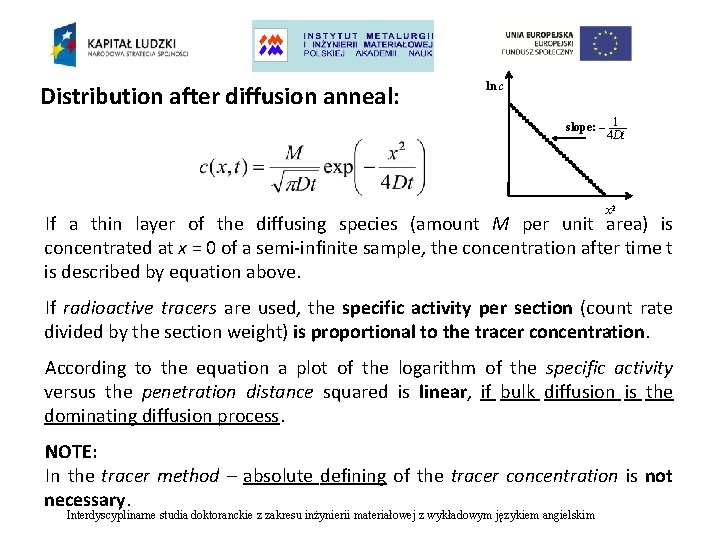
Distribution after diffusion anneal: ln c slope: – 1 4 Dt x 2 If a thin layer of the diffusing species (amount M per unit area) is concentrated at x = 0 of a semi-infinite sample, the concentration after time t is described by equation above. If radioactive tracers are used, the specific activity per section (count rate divided by the section weight) is proportional to the tracer concentration. According to the equation a plot of the logarithm of the specific activity versus the penetration distance squared is linear, if bulk diffusion is the dominating diffusion process. NOTE: In the tracer method – absolute defining of the tracer concentration is not necessary. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Need to know: Ø Grain boundaries in polycrystalline sample act as diffusion short circuits with enhanced mobility of atoms. Grain boundaries usually cause ‘grain boundary tail’ in the deeper penetrating part of the profile. In this tail region the concentration of the diffuser is enhanced with respect to mere bulk diffusion. Ø Evaporation losses of the tracer cause deviation in the near-surface region. Ø Stable isotopes can be used as tracers as well. Then SIMS is an appropriate technique for depth profiling. Contrary to self-diffusion studies by radiotracer experiments, in the case of stable tracers the natural abundance of the stable isotope in the matrix limits the concentration range of the diffusion profile. Highly enriched isotopes should be used. Ø In some cases several tracer isotopes of the same element are available with different isotopic masses. Differences between the isotopic masses lead to isotope effects in diffusion. However, the differences between diffusivities of two different isotopes of the same element are usually a few percent only. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

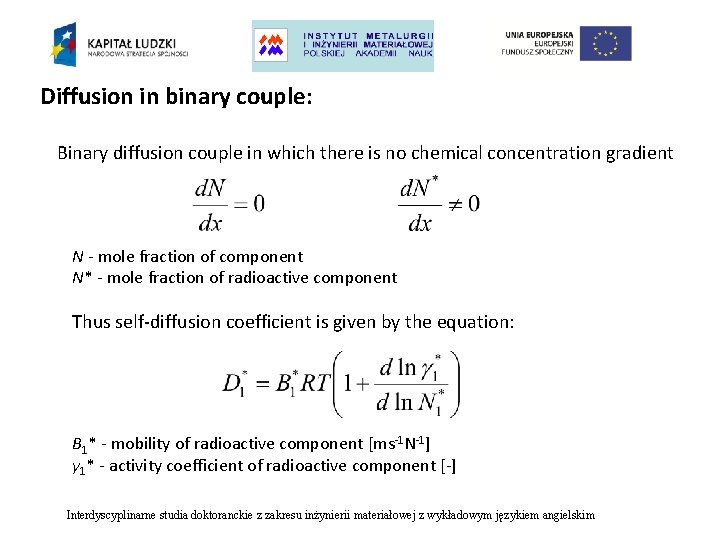
Diffusion in binary couple: Binary diffusion couple in which there is no chemical concentration gradient N - mole fraction of component N* - mole fraction of radioactive component Thus self-diffusion coefficient is given by the equation: B 1* - mobility of radioactive component [ms-1 N-1] γ 1* - activity coefficient of radioactive component [-] Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

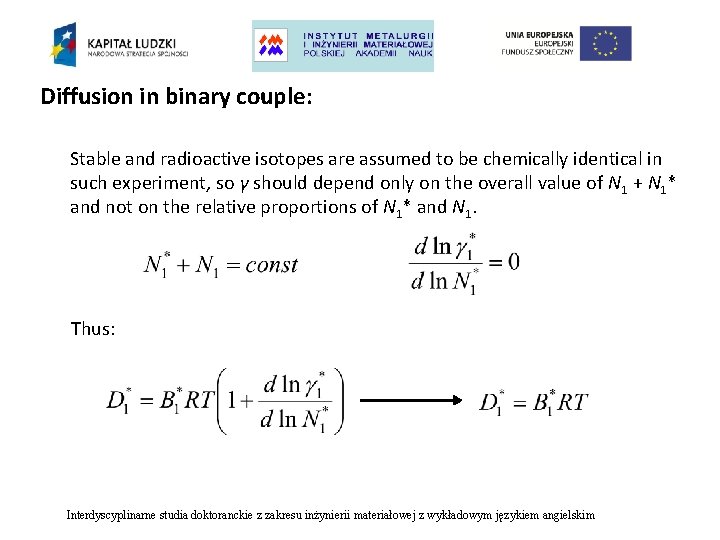
Diffusion in binary couple: Stable and radioactive isotopes are assumed to be chemically identical in such experiment, so γ should depend only on the overall value of N 1 + N 1* and not on the relative proportions of N 1* and N 1. Thus: Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

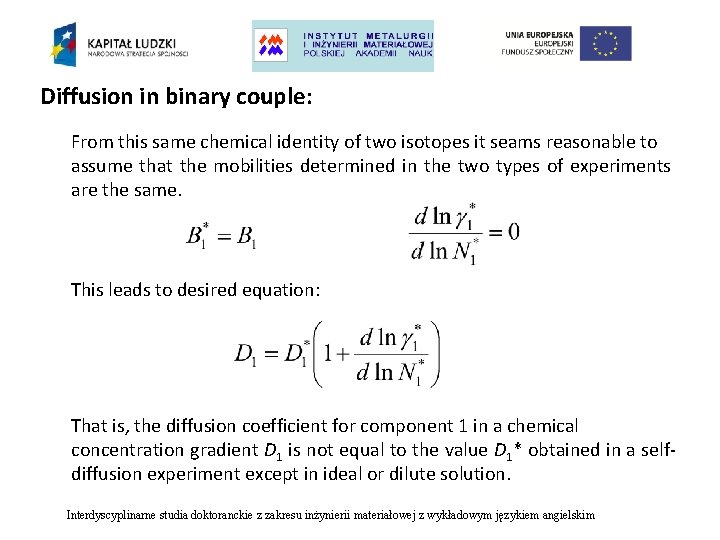
Diffusion in binary couple: From this same chemical identity of two isotopes it seams reasonable to assume that the mobilities determined in the two types of experiments are the same. This leads to desired equation: That is, the diffusion coefficient for component 1 in a chemical concentration gradient D 1 is not equal to the value D 1* obtained in a selfdiffusion experiment except in ideal or dilute solution. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

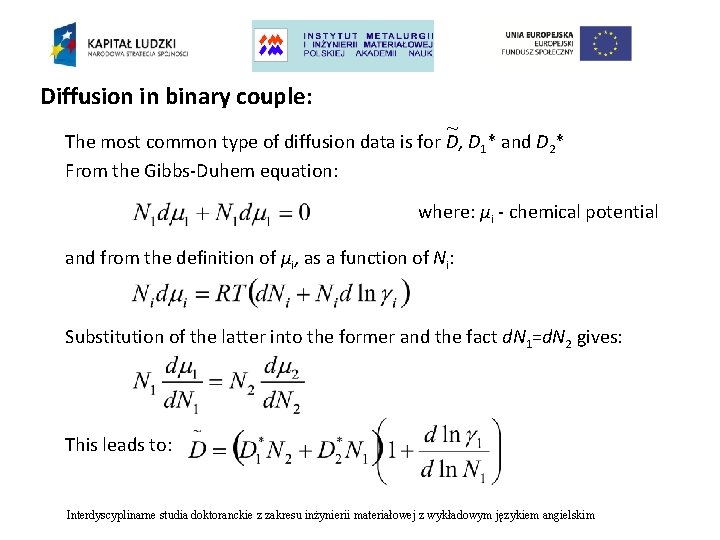
Diffusion in binary couple: ~ The most common type of diffusion data is for D, D 1* and D 2* From the Gibbs-Duhem equation: where: μi - chemical potential and from the definition of μi, as a function of Ni: Substitution of the latter into the former and the fact d. N 1=d. N 2 gives: This leads to: Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

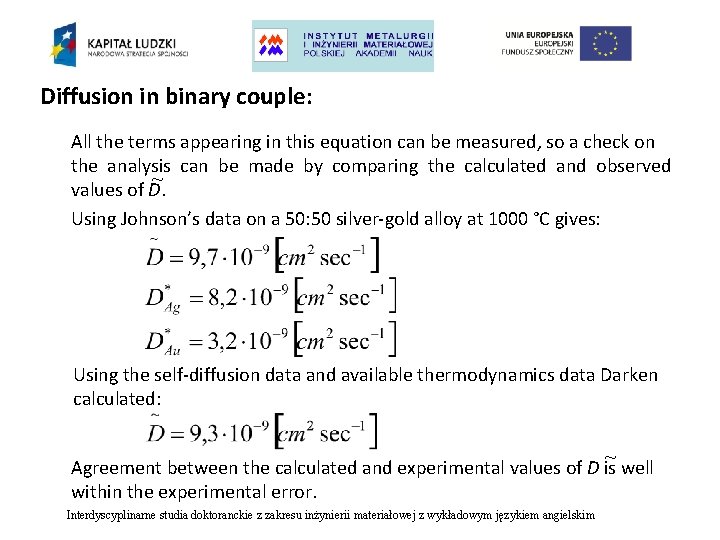
Diffusion in binary couple: All the terms appearing in this equation can be measured, so a check on the analysis can be made by comparing the calculated and observed ~ values of D. Using Johnson’s data on a 50: 50 silver-gold alloy at 1000 °C gives: Using the self-diffusion data and available thermodynamics data Darken calculated: ~ Agreement between the calculated and experimental values of D is well within the experimental error. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Diffusion in multiphase systems 6. Wagner’s integral interdiffusion coefficient Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

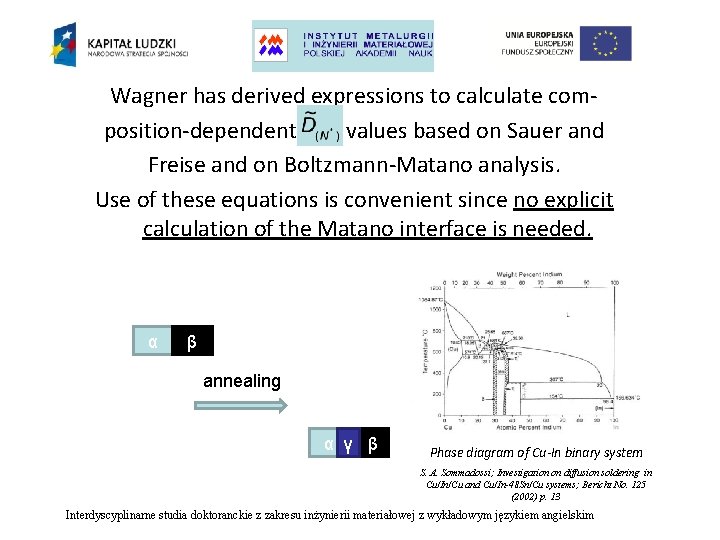
Wagner has derived expressions to calculate composition-dependent values based on Sauer and Freise and on Boltzmann-Matano analysis. Use of these equations is convenient since no explicit calculation of the Matano interface is needed. α β annealing α γ β Phase diagram of Cu-In binary system S. A. Sommadossi; Investigation on diffusion soldering in Cu/In/Cu and Cu/In-48 Sn/Cu systems; Bericht No. 125 (2002) p. 13 Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

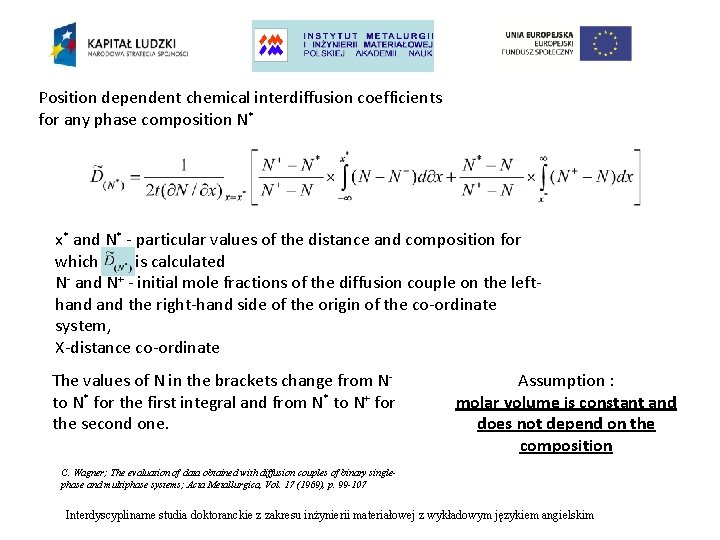
Position dependent chemical interdiffusion coefficients for any phase composition N* x* and N* - particular values of the distance and composition for which is calculated N- and N+ - initial mole fractions of the diffusion couple on the lefthand the right-hand side of the origin of the co-ordinate system, X-distance co-ordinate The values of N in the brackets change from Nto N* for the first integral and from N* to N+ for the second one. Assumption : molar volume is constant and does not depend on the composition C. Wagner; The evaluation of data obtained with diffusion couples of binary singlephase and multiphase systems; Acta Metallurgica, Vol. 17 (1969), p. 99 -107 Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

This equation is valid for all types of diffusion couples where either a single phase (solid solution or intermediate phase) or multiple phases appear in the diffusion zone. With the help of this equation one may obtain, in principle, values of as a function of composition for all the phases (present in the diffusion zone) from a single diffusion experiment. For better accuracy, however, Wagner has recommended to make separate runs for different composition ranges. S. P. Garg et al. , Thermodynamic interdiffusion coeffcient in binary systems with intermediate phases; Intermetallics 7 (1999) p. 901 -908. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

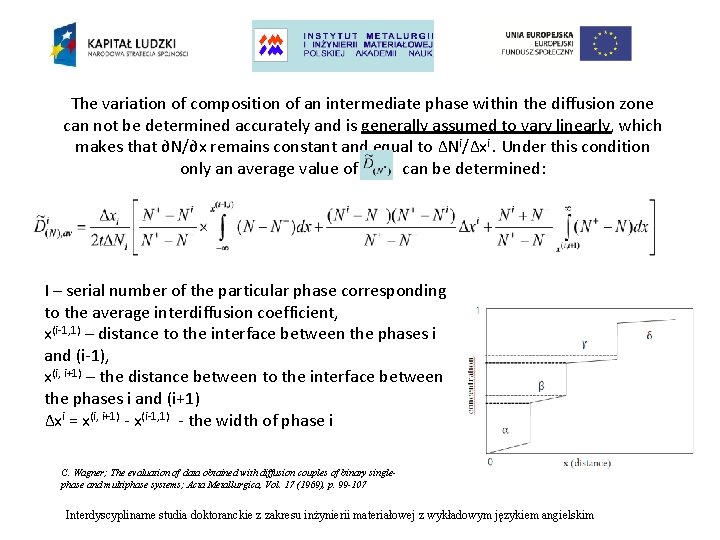
The variation of composition of an intermediate phase within the diffusion zone can not be determined accurately and is generally assumed to vary linearly, which makes that ∂N/∂x remains constant and equal to ∆Ni/∆xi. Under this condition only an average value of can be determined: I – serial number of the particular phase corresponding to the average interdiffusion coefficient, x(i-1, 1) – distance to the interface between the phases i and (i-1), x(i, i+1) – the distance between to the interface between the phases i and (i+1) ∆xi = x(i, i+1) - x(i-1, 1) - the width of phase i C. Wagner; The evaluation of data obtained with diffusion couples of binary singlephase and multiphase systems; Acta Metallurgica, Vol. 17 (1969), p. 99 -107 Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

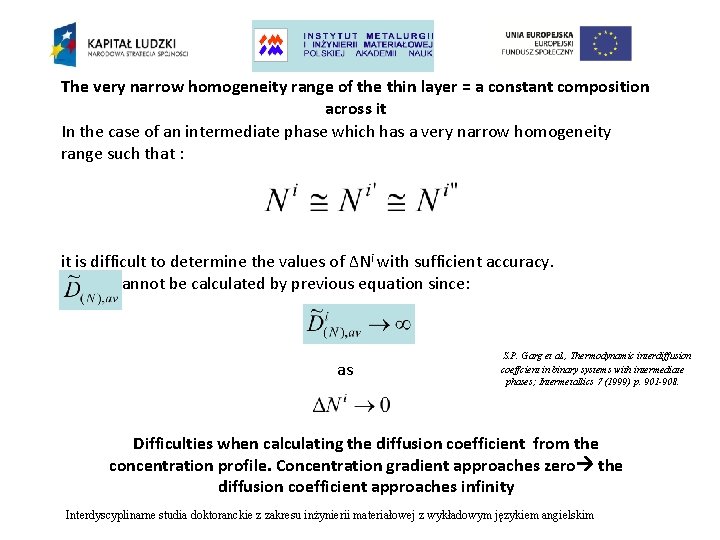
The very narrow homogeneity range of the thin layer = a constant composition across it In the case of an intermediate phase which has a very narrow homogeneity range such that : it is difficult to determine the values of ∆Ni with sufficient accuracy. cannot be calculated by previous equation since: as S. P. Garg et al. , Thermodynamic interdiffusion coeffcient in binary systems with intermediate phases; Intermetallics 7 (1999) p. 901 -908. Difficulties when calculating the diffusion coefficient from the concentration profile. Concentration gradient approaches zero the diffusion coefficient approaches infinity Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

Wagner: o made an assumption that the concentrations at the interfaces are constant and equal to their equilibrium values o integrated the interdiffusion coefficient over distances coordinates to avoid determination of the concentration gradient o found relationship between interdiffusion coefficient and the parabolic growth rate constant S. A. Sommadossi; Investigation on diffusion soldering in Cu/In/Cu and Cu/In-48 Sn/Cu systems; Bericht No. 125 (2002) p. 13 -27; Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

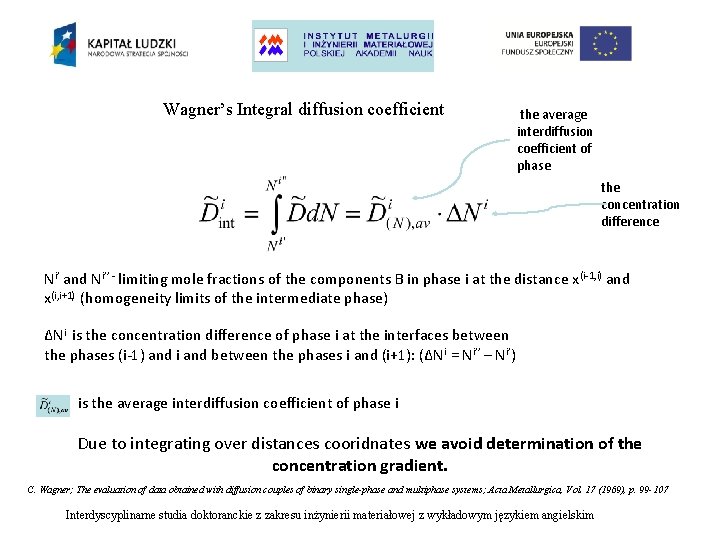
Wagner’s Integral diffusion coefficient the average interdiffusion coefficient of phase the concentration difference Ni’ and Ni’’ - limiting mole fractions of the components B in phase i at the distance x (i-1, i) and x(i, i+1) (homogeneity limits of the intermediate phase) ΔNi is the concentration difference of phase i at the interfaces between the phases (i-1) and i and between the phases i and (i+1): (ΔNi = Ni’’ – Ni’) is the average interdiffusion coefficient of phase i Due to integrating over distances cooridnates we avoid determination of the concentration gradient. C. Wagner; The evaluation of data obtained with diffusion couples of binary single-phase and multiphase systems; Acta Metallurgica, Vol. 17 (1969), p. 99 -107 Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

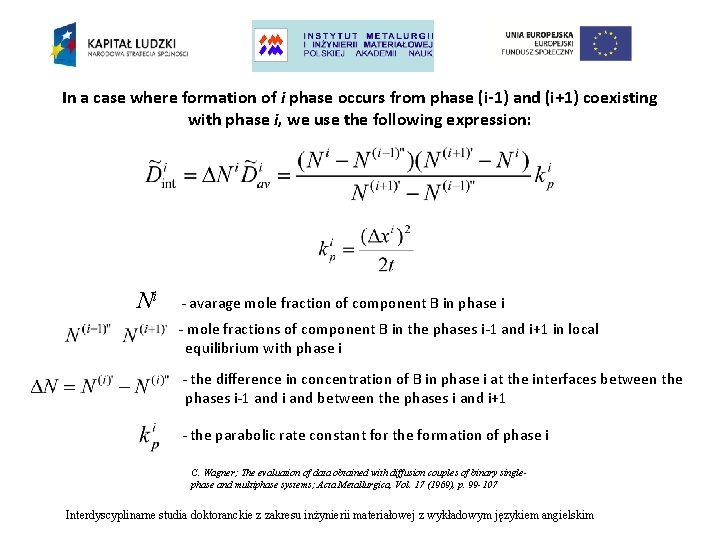
In a case where formation of i phase occurs from phase (i-1) and (i+1) coexisting with phase i, we use the following expression: Ni - avarage mole fraction of component B in phase i - mole fractions of component B in the phases i-1 and i+1 in local equilibrium with phase i - the difference in concentration of B in phase i at the interfaces between the phases i-1 and i and between the phases i and i+1 - the parabolic rate constant for the formation of phase i C. Wagner; The evaluation of data obtained with diffusion couples of binary singlephase and multiphase systems; Acta Metallurgica, Vol. 17 (1969), p. 99 -107 Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

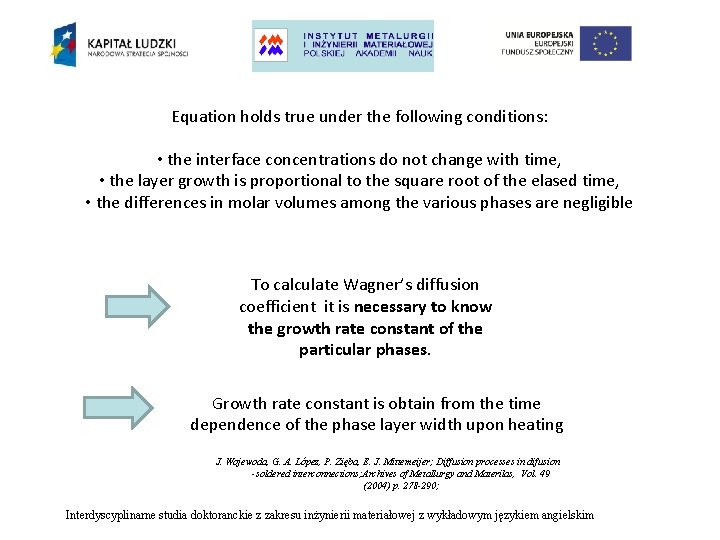
Equation holds true under the following conditions: • the interface concentrations do not change with time, • the layer growth is proportional to the square root of the elased time, • the differences in molar volumes among the various phases are negligible To calculate Wagner’s diffusion coefficient it is necessary to know the growth rate constant of the particular phases. Growth rate constant is obtain from the time dependence of the phase layer width upon heating J. Wojewoda, G. A. López, P. Zięba, E. J. Mittemeijer; Diffusion processes in difusion -soldered interconnections; Archives of Metallurgy and Materilas, Vol. 49 (2004) p. 278 -290; Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

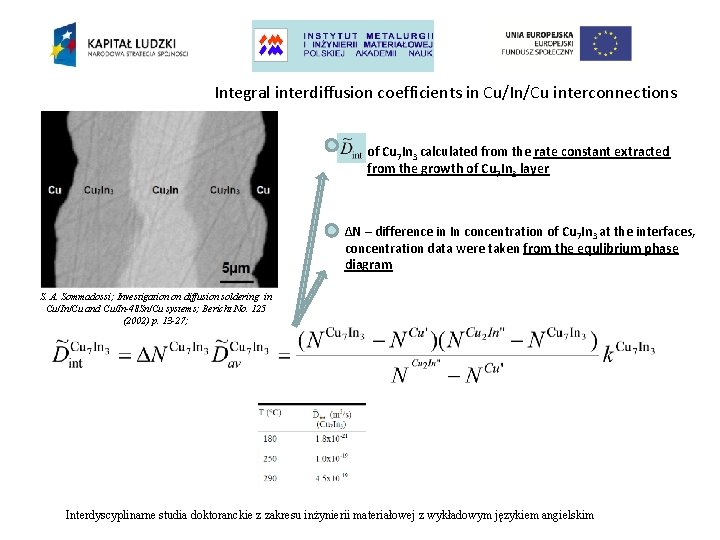
Integral interdiffusion coefficients in Cu/In/Cu interconnections of Cu 7 In 3 calculated from the rate constant extracted from the growth of Cu 7 In 3 layer ∆N – difference in In concentration of Cu 7 In 3 at the interfaces, concentration data were taken from the equlibrium phase diagram S. A. Sommadossi; Investigation on diffusion soldering in Cu/In/Cu and Cu/In-48 Sn/Cu systems; Bericht No. 125 (2002) p. 13 -27; Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim

1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. References http: //www. doitpoms. ac. uk/tlplib/diffusion/index. php; http: //dmseg 5. case. edu/classes/emse 201/overheads/Diffusio 1. pdf; R. Tilley, Understending solids, John Wiley&Sons Ltd 2004; D. A. Porter, K. E. Easterling, M. Y. Sherif, Phase Transformations in Metals and Alloys, CRC Press, Boca Raton-London. New York 2009; Z. Kędzierski, Przemiany fazowe w układach skondensowanych, Uczelniane Wydawnictwa Naukowo-Techniczne, Kraków 2003; S. Prowans, Struktura stopów, Wydawnictwo Naukowe PWN, Warszawa 2000; E. Metin, O. T. Inal, A. D. Roming: ‘Solutions to Multiphase Diffusion in Binary Metal Interstitial Systems’, Metallurgical and Materials Transactions A, Vol. 36 A, (2005) 1407 -1415; J. Drapala et al. / Mathematics and Computers in Simulation 80 (2010) 1520– 1535; http: //www. calphad. com/iron-manganese. html; P. Aloke, The Kirkendall Effect in Solid State Diffusion, Technische Universiteit Eindhoven (2004); J. Karger, P. Heitjans, R. Haberlandt, Diffusion in Condensed Matter, Vieweg & Sohn Verlagsgesellschaft mb. H, Braunschweig/Wiesbaden, 1998; P. G. Shewmon, Diffusion in Solids, Mc. Graw-Hill Book Company Inc. , New York, 1963; D. A. Porter, K. E. Easterling, M. Y. Sherif, Phase Transformations in Metals and Alloys, CRC Press Taylor & Francis Group, Boca Raton, 2009; C. Wagner; The evaluation of data obtained with diffusion couples of binary single-phase and multiphase systems; Acta Metallurgica, Vol. 17 (1969), p. 99 -107; J. Wojewoda, G. A. López, P. Zięba, E. J. Mittemeijer; Diffusion processes in difusion-soldered interconnections; Archives of Metallurgy and Materilas, Vol. 49 (2004) p. 278 -290; S. A. Sommadossi; Investigation on diffusion soldering in Cu/In/Cu and Cu/In-48 Sn/Cu systems; Bericht No. 125 (2002) p. 13 -27; S. P. Garg et al. , Thermodynamic interdiffusion coefficient in binary systems with intermediate phases; Intermetallics 7 (1999) p. 901 -908. Interdyscyplinarne studia doktoranckie z zakresu inżynierii materiałowej z wykładowym językiem angielskim
 Multiphase buck
Multiphase buck Antoine equation
Antoine equation Multiphase iterative design
Multiphase iterative design Agar mpfm
Agar mpfm Two film theory
Two film theory Multiphase flow meter
Multiphase flow meter Jagoda różycka
Jagoda różycka Jagoda prądzyńska wiek
Jagoda prądzyńska wiek Ta nam
Ta nam Metoda krakowska
Metoda krakowska Jagoda egeland
Jagoda egeland Swabt
Swabt Relocation and expansion diffusion
Relocation and expansion diffusion Decision support systems and intelligent systems
Decision support systems and intelligent systems Principles of complex systems for systems engineering
Principles of complex systems for systems engineering Embedded systems vs cyber physical systems
Embedded systems vs cyber physical systems Elegant systems
Elegant systems Howard county young authors contest
Howard county young authors contest In text citation multiple authors
In text citation multiple authors Whats authors purpose
Whats authors purpose What is the author's claim in this passage
What is the author's claim in this passage Topic 15 periods authors and genres
Topic 15 periods authors and genres What is the author attitude towards the subject
What is the author attitude towards the subject Name and affiliation
Name and affiliation Read the veldt
Read the veldt In text citation chicago multiple authors
In text citation chicago multiple authors Important of symbols
Important of symbols Something concrete that stands for something abstract
Something concrete that stands for something abstract Author et al
Author et al How to cite many authors
How to cite many authors Why do authors use hypophora
Why do authors use hypophora Author's tone
Author's tone Postmodernism definition
Postmodernism definition Definition of authors purpose
Definition of authors purpose How to cite many authors
How to cite many authors How to cite multiple authors
How to cite multiple authors Parenthetical citation for multiple authors
Parenthetical citation for multiple authors Imagery in oranges by gary soto
Imagery in oranges by gary soto Types of author's bias
Types of author's bias English writer name
English writer name Examples of foreshadowing
Examples of foreshadowing The girl ran her hands on a soft satin fabric
The girl ran her hands on a soft satin fabric Direct quotation examples
Direct quotation examples Apa authors note
Apa authors note Author's argument
Author's argument Authors by initials
Authors by initials Famous black author
Famous black author Tones of authors
Tones of authors Authors purpose pie
Authors purpose pie To entertain
To entertain Flocabulary point of view
Flocabulary point of view Author's purpose powerpoint
Author's purpose powerpoint Dialogue quote vs flow quote
Dialogue quote vs flow quote Armen yuri gasparyan
Armen yuri gasparyan How to cite 2 authors apa in-text
How to cite 2 authors apa in-text Font apa
Font apa In text referencing for multiple authors
In text referencing for multiple authors In text citation apa 2 authors
In text citation apa 2 authors Et al in text citations
Et al in text citations How to write et al in apa
How to write et al in apa Apa intext citation 3 authors
Apa intext citation 3 authors How do you in-text cite two authors in apa?
How do you in-text cite two authors in apa? In text citation apa
In text citation apa Two authors in text citation apa
Two authors in text citation apa Apa author name
Apa author name