Diffusion Diffusion The motion of atoms through matter



























- Slides: 44

Diffusion

• Diffusion: The motion of atoms through matter • Diffusion Couple: An assembly of two materials in such intimate contact that each diffuse into each other • Ficks First law of Diffusion: • The flux of diffusing species is proportional to the concentration gradient

• Fick second law of diffusion: • The rate of change of composition is proportional to the second derivate of the concentration • Grain Boundary Diffusion: Atoms move along the grain boundary • Self Diffusion: The migration of atoms in pure materials

• Surface diffusion: • Atomic migration along the surface of a phase e. g along a solid-vapor interface • Volume diffusion • Atomic migration through the bulk of the material

Types of Diffusion • Kinetic barrier to the movement of an atom through a solid lattice is greater than that to movement through a liquid or a gas. • This is reflected in the higher activation enthalpy or • Energy Q that is necessary for volume diffusion through a solid than through a liquid or gas. Volume diffusion is clearly important for a case like the one demonstrated in Figure 5. 2.

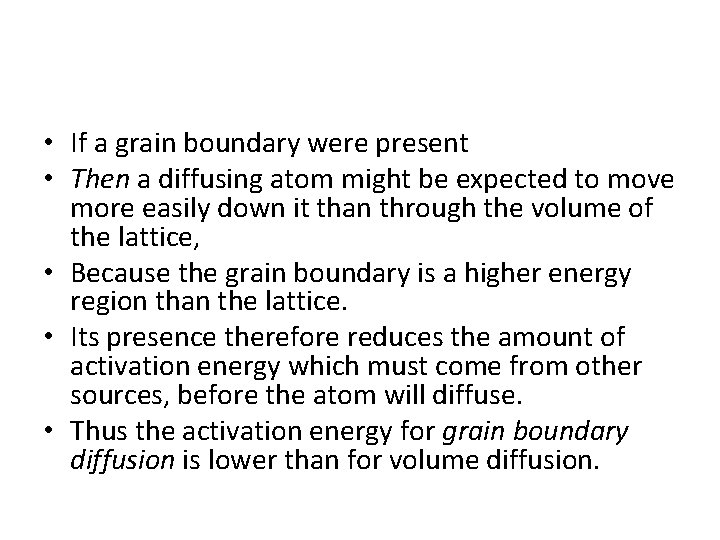
• If a grain boundary were present • Then a diffusing atom might be expected to move more easily down it than through the volume of the lattice, • Because the grain boundary is a higher energy region than the lattice. • Its presence therefore reduces the amount of activation energy which must come from other sources, before the atom will diffuse. • Thus the activation energy for grain boundary diffusion is lower than for volume diffusion.

Three types of Diffusion • 1) Volume diffusion: Through the lattice required activation energy Qvol • 2)Grain Boundary Diffusion: Along the grain boundary activation energy QG. B • 3) Surface Diffusion: The atoms would be transported inward by surface diffusion required activation energy QSurf

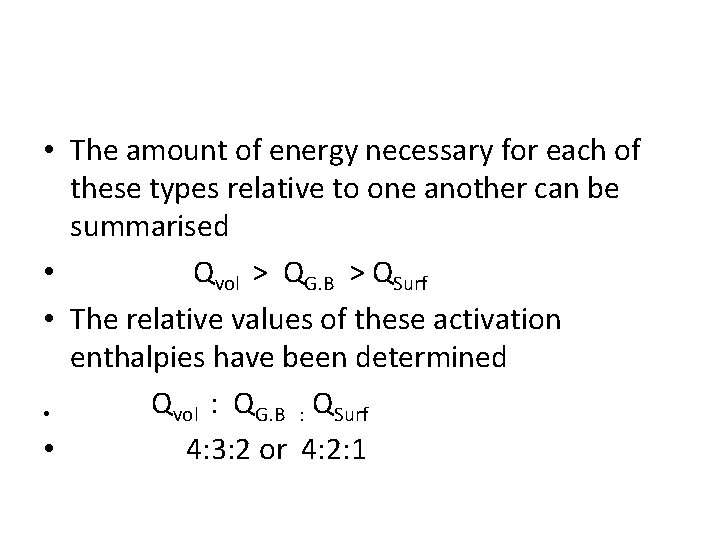
• The amount of energy necessary for each of these types relative to one another can be summarised • Qvol > QG. B > QSurf • The relative values of these activation enthalpies have been determined Qvol : QG. B : QSurf • • 4: 3: 2 or 4: 2: 1

• Three coefficients of diffusion may be arranged • Dsurf > DG. B > Dvol • The relative importance of three types of diffusion in actual process: • Does not depend on the diffusion coefficient alone. • The amount of material transported by any of the three types of diffusion is given by (Fick’s First Law).

• For the same composition gradient, the amount of material transported depends also on • The effective area through which atoms diffuse. • The effective “thickness” of a grain boundary or of a surface diffusion path is assumed to be several atom spacing's (Approximately IO-7 cm), • The areas of these paths are very small compared to those of the volume diffusion path

• It is suggested that only at low temperatures, • When the value of Dvol has dropped far below that of the others, • Grain boundaries and surfaces become really important as mass transport paths. • It is also possible to show that grain boundary diffusion competes with volume diffusion only in very fine-grained material,

• It is also possible to show that grain boundary diffusion competes with volume diffusion only in very fine-grained material, • Depending on the ratio of Dg. b to Dvol. • In processes such as sintering and oxidation, grain boundary diffusion and surface diffusion are very important.

ATOMIC MECHANISMS OF VOLUME DIFFUSION • The mechanics of the motion of atoms or molecules in fluids are relatively well known • In most gases the molecules move in straight paths until they collide. • The change in path resulting from these collisions may be estimated. • The cumulative motion of these molecules may then be predicted from the collision parameters, • This procedure yields the diffusive properties of the gas in question.

• The mechanism of diffusion in solids is less clear • Somehow an atom in a certain lattice site is transferred to an adjacent site. • This is the basic step in the diffusion process. • The actual method by which this transfer occurs, • However, has not been unambiguously demonstrated.

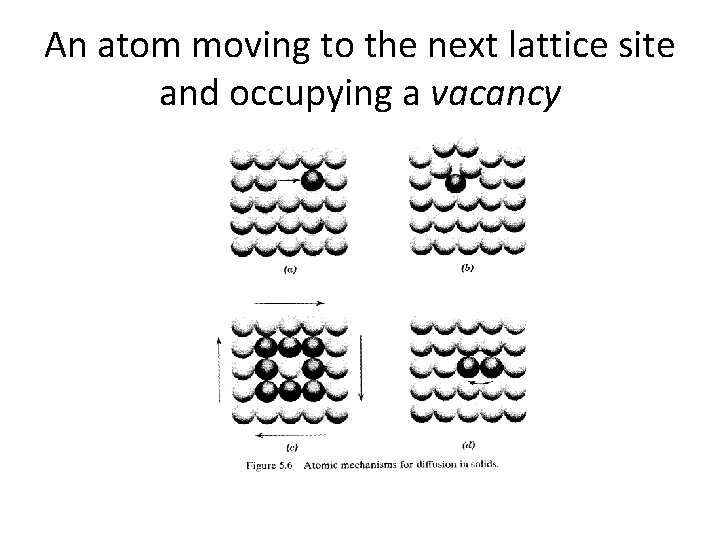
An atom moving to the next lattice site and occupying a vacancy

• Figure 5. 6 a shows an atom moving to the next lattice site and occupying a vacancy there. • In Figure 5. 6 b an atom moves out of its lattice and becomes an interstitial atom which is free to move. • In Figure 5. 6 c atoms in a ring simultaneously move to adjacent lattice sites. • In Figure 5. 6 d two atoms change places directly.

Vacancy Mechanism • The vacancy mechanism currently appears to be the most probable one in self-diffusion • Also in diffusion of substitutional solid solution elements and ions in metals and ceramics. • If vacancies are already present, • The activation energy for diffusion in this kind of situation is only that required for an atom to part with one set of near neighbors and move into a vacant site among another set. • Reasonable agreement has been found between observed diffusivities and calculations based on this model

• The interstitial mechanism is important in two cases. • A solute atom, when small enough to dissolve interstitially, moves most readily by this mechanism. • This occurs particularly when carbon, nitrogen, oxygen, and hydrogen dissolve and diffuse in metals, and ions of alkali metals and various gases in silicate glasses and vitreous materials. • In these cases, diffusivities are quite high at surprisingly low temperatures

• Interstitial diffusion also occurs in materials subjected to neutron irradiation. • High-energy neutrons supply the energy necessary to “knock out” an atom from a lattice site • Into an interstitial position from where it can move freely.

Substitutional solid solutions • In all other substitutional solid solutions, • The amount of energy required to place an atom in an interstitial site, by thermal excitation, • Is too great for interstitial diffusion to occur at a significant rate.

• The direct interchange mechanism is unlikely because it too requires a high activation energy. • The ring mechanism has a sufficiently low activation energy • So it is to be operative in some situations and to explain some experimental observations


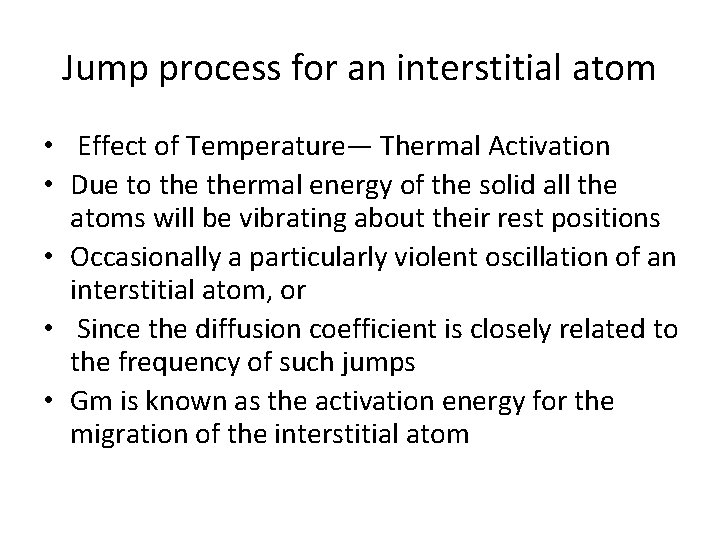
Jump process for an interstitial atom • Effect of Temperature— Thermal Activation • Due to thermal energy of the solid all the atoms will be vibrating about their rest positions • Occasionally a particularly violent oscillation of an interstitial atom, or • Since the diffusion coefficient is closely related to the frequency of such jumps • Gm is known as the activation energy for the migration of the interstitial atom

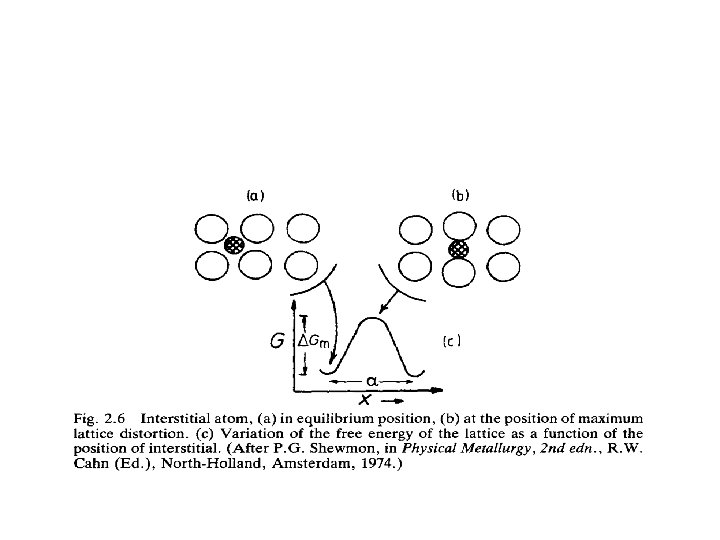
• The rest positions of the interstitial atoms are positions of minimum potential energy. • In order to move an interstitial atom to an adjacent interstice • The atoms of the parent lattice must be forced apart into higher energy positions as shown in Fig. 2. 6 b. • The work that must be done to accomplish this process causes an increase in the free energy of the system by Gm

• Gm is known as the activation energy for the migration of the interstitial atom. • In any system in thermal equilibrium the atoms are constantly colliding with one another and changing their vibrational energy.

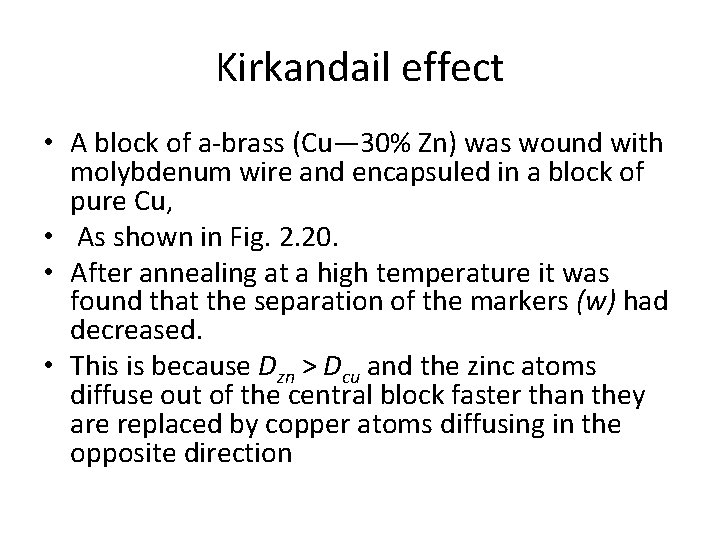
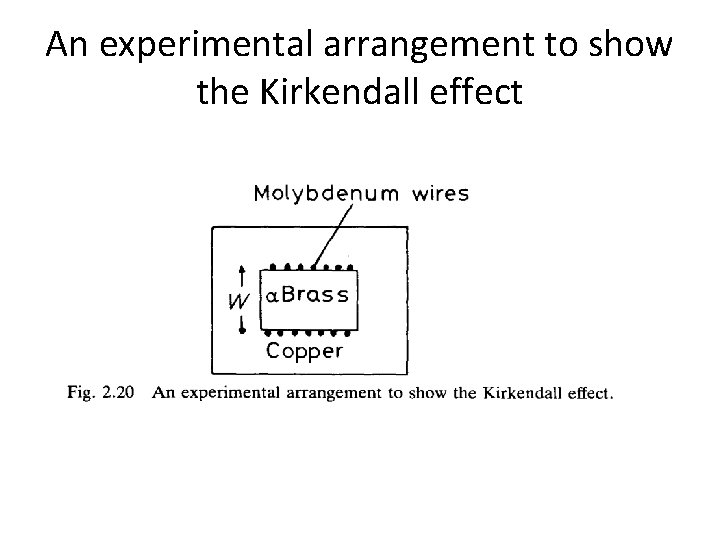
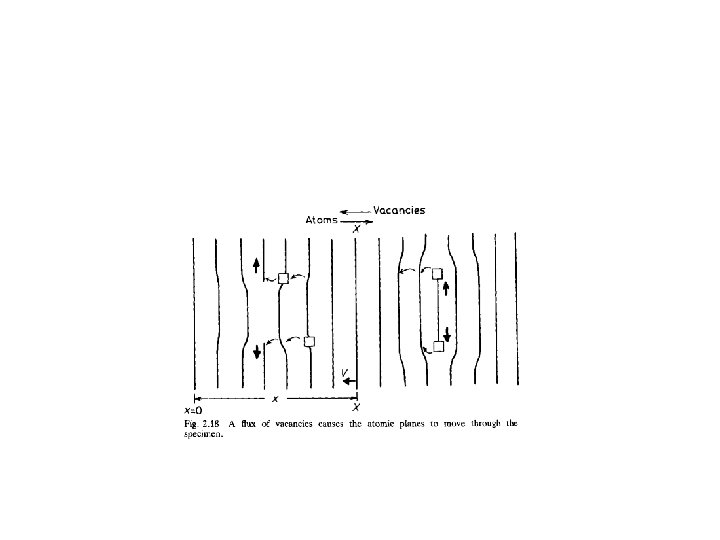
Kirkandail effect • A block of a-brass (Cu— 30% Zn) was wound with molybdenum wire and encapsuled in a block of pure Cu, • As shown in Fig. 2. 20. • After annealing at a high temperature it was found that the separation of the markers (w) had decreased. • This is because Dzn > Dcu and the zinc atoms diffuse out of the central block faster than they are replaced by copper atoms diffusing in the opposite direction

An experimental arrangement to show the Kirkendall effect

• Similar effects have since been demonstrated in many other alloy systems. • In general it is found that in any given couple, atoms with the lower melting point possess a higher D. • The exact value of D, however, varies with the composition of the alloy. • Thus in Cu—Ni alloys Dcu and DNi and D are all composition dependent, • increases as Xcu , increases.

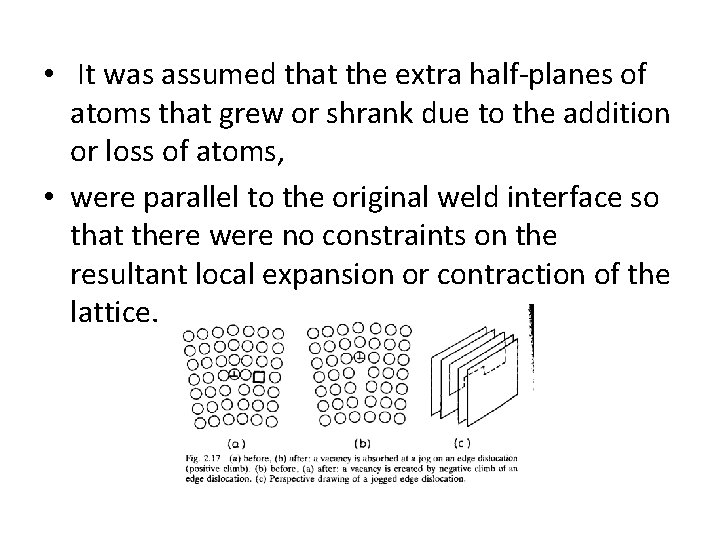
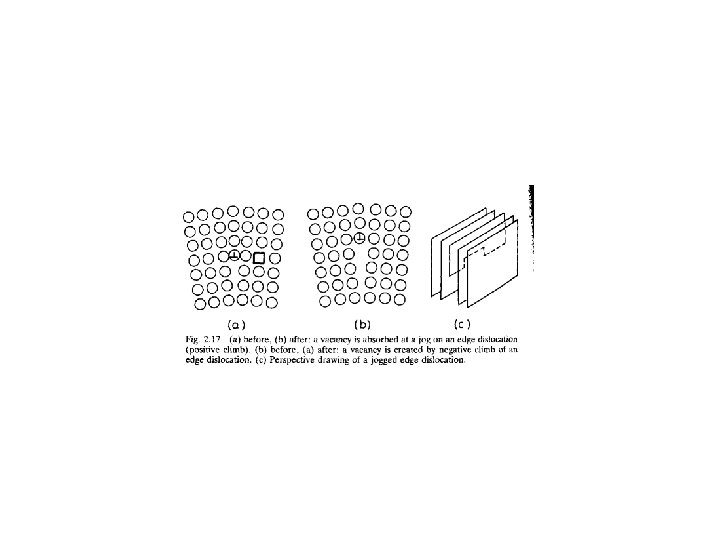
• It was assumed that the extra half-planes of atoms that grew or shrank due to the addition or loss of atoms, • were parallel to the original weld interface so that there were no constraints on the resultant local expansion or contraction of the lattice.

• In practice, however, these planes can be oriented in many directions • And the lattice will also try to expand or contract parallel to the weld interface. • Such volume changes are restricted by the surrounding material which resulted that • Two-dimensional compressive stresses develop in regions where vacancies are created,

• While tensile stresses arise in regions where vacancies are destroyed. • These stress fields can even induce plastic deformation • Resulting in microstructures characteristic of hot deformation.

• Vacancies are not necessarily all annihilated at dislocations, • But can also be absorbed by internal boundaries and free surfaces. • However, those not absorbed at dislocations mainly agglomerate to form holes or voids in the lattice. • Void nucleation is difficult because it requires the creation of a new surface and • it is generally believed that voids are heterogeneously nucleated at impurity particles

• The tensile stresses that arise in conjunction with vacancy destruction • Can also play a role in the nucleation of voids. • When voids are formed the Equations derived above cannot be used without modification. In concentrated alloys the experimentally determined values of 15, DA and DB are also found to show the same form of temperature dependence as all



Atomic Mobility • Fick’s first law is based on the assumption that diffusion eventually stops, when equilibrium is reached, • Or when the concentration is the same everywhere. • Strictly speaking this situation is never true in practice • Because real materials always contain lattice defects • such as grain boundaries, phase boundaries and dislocations.

• Some atoms can lower their free energies if they migrate to such defects and • And at equilibrium’ • Their concentrations will be higher in the vicinity of the defect than in the matrix • Diffusion in the vicinity of these defects is therefore affected by • the concentration gradient and the gradient of the interaction energy

• Fick’s law alone is insufficient to describe how the concentration will vary with distance and time. e. g • Consider the case of a solute atom that is too big or too small in comparison to the space available in the solvent lattice. • The potential energy of the atom will then be relatively high due to the strain in the surrounding matrix.

• However, this strain energy can be reduced • If the atom is located in a position • Where it better matches the space available, e. g. near dislocations and in boundaries, • where the matrix is already distorted.

• Segregation of atoms to grain boundaries, interfaces and dislocations is of great technological importance. • For example the diffusion of carbon or nitrogen to dislocations in mild steel is responsible for strain ageing and blue brittleness. • The segregation of impurities such as Sb, Sn, P and As to grain boundaries in low-alloy steels produces temper embrittlement.

• Segregation to grain boundaries affects the mobility of the boundary and • Therefore, has pronounced effects on recrystallization, texture and grain growth. • Similarly the rate at which phase transformations occur is sensitive to segregation at dislocations and interfaces

• The problem of atom migration can be solved by considering thermodynamic condition for equilibrium; • Namely that the chemical potential of an atom must be the same everywhere. • Diffusion continues in fact until this condition is satisfied. • Therefore it seems reasonable to suppose that in general the flux of atoms at any point in the lattice is proportional to the chemical potential gradient. • Fick’s first law is merely a special case of this more general approach.

• Fick’s first law is merely a special case of this more general approach. • An alternative way to describe a flux of atoms is to consider • a net drift velocity (v) superimposed on the random jumping motion of each diffusing atom. • The drift velocity is simply related to the diffusive flux via the equation

• Since atoms always migrate so as to remove differences in chemical potential • it is reasonable to suppose that the drift velocity is proportional to the local chemical potential gradient, i. e.