Diffusion Diffusion movement of particles from an area



- Slides: 21

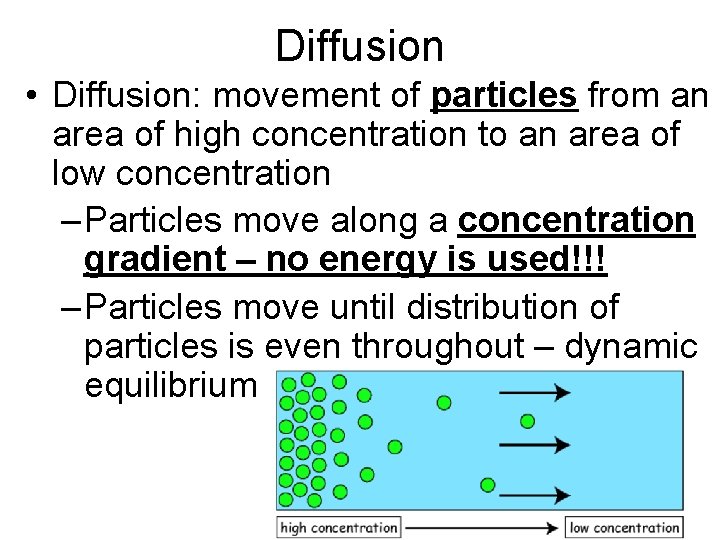
Diffusion • Diffusion: movement of particles from an area of high concentration to an area of low concentration – Particles move along a concentration gradient – no energy is used!!! – Particles move until distribution of particles is even throughout – dynamic equilibrium

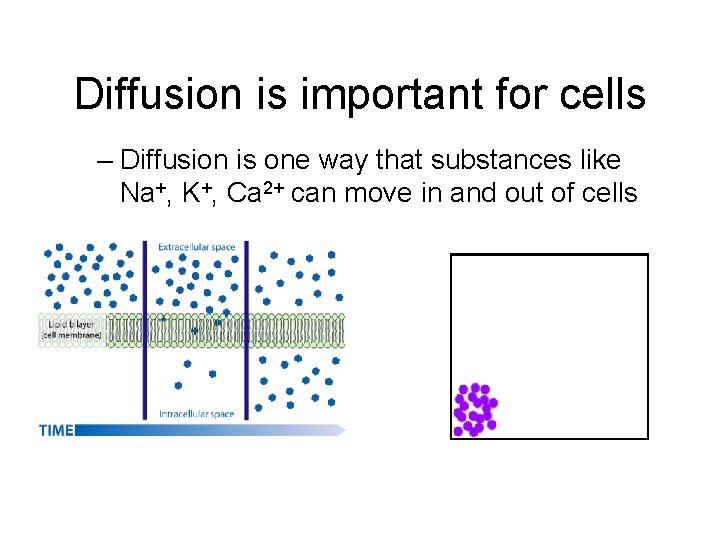
Diffusion is important for cells – Diffusion is one way that substances like Na+, K+, Ca 2+ can move in and out of cells

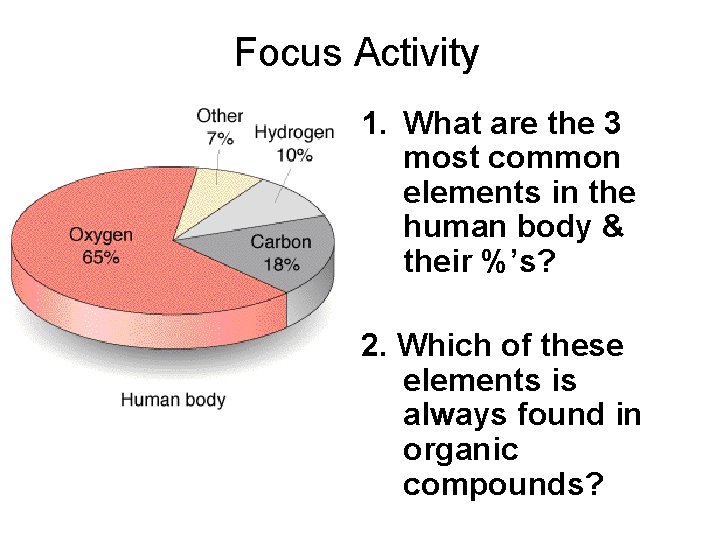
Focus Activity 1. What are the 3 most common elements in the human body & their %’s? 2. Which of these elements is always found in organic compounds?

Biochemistry

Notes Chapter 6. 3 – Life Substances and Organic Chemistry • Organic chemistry is the study of Carbonbased compounds. • All living things are composed of organic compounds.

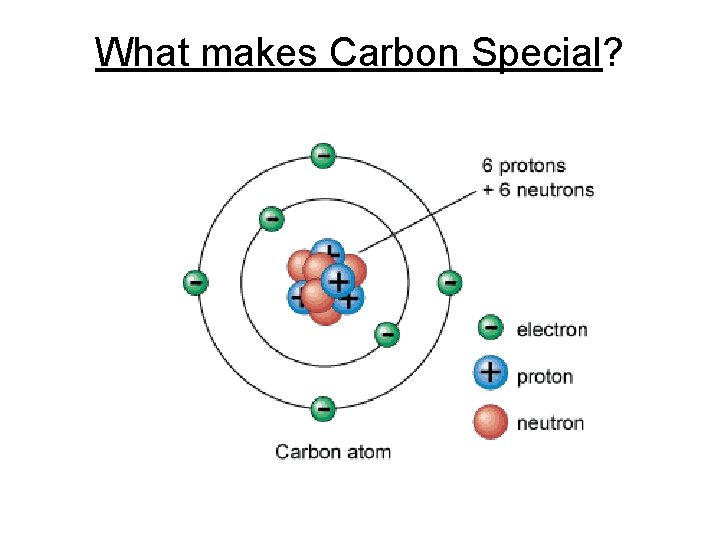
What makes Carbon Special?

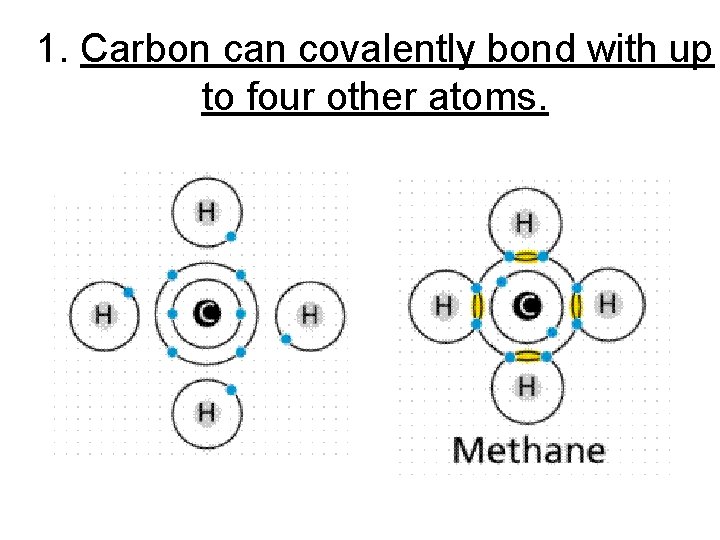
1. Carbon can covalently bond with up to four other atoms.

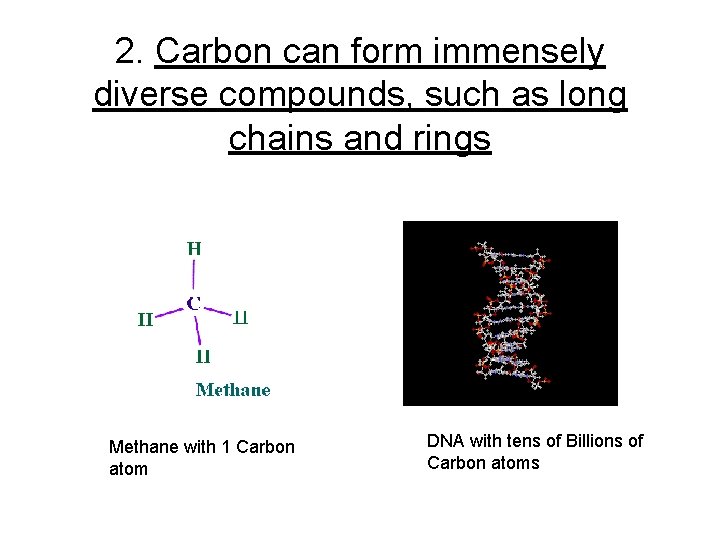
2. Carbon can form immensely diverse compounds, such as long chains and rings Methane with 1 Carbon atom DNA with tens of Billions of Carbon atoms

There are 4 Fundamental Organic Molecules used in our bodies • 1. Carbohydrates (sugars and starches) • 2. Lipids (fats, waxes, oils, steroid hormones) • 3. Proteins – (enzymes, muscles tissue, cell structures) • 4. Nucleic Acids – (DNA and RNA which are our genetic material)

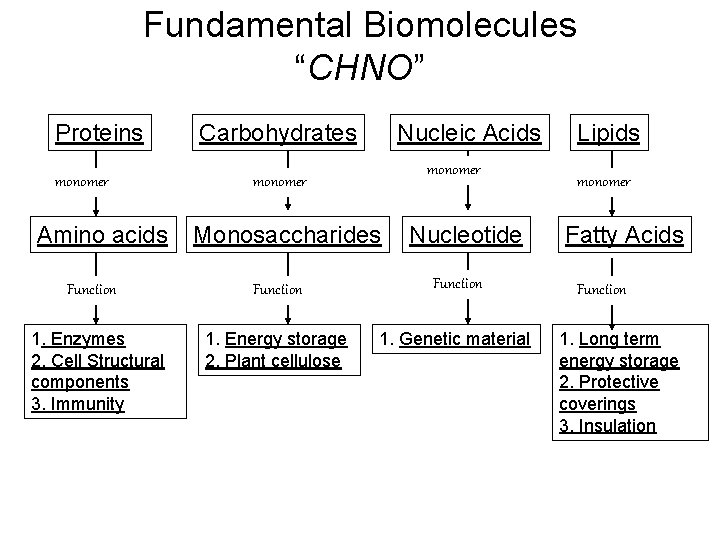
Fundamental Biomolecules “CHNO” Proteins monomer Amino acids Function 1. Enzymes 2. Cell Structural components 3. Immunity Carbohydrates Nucleic Acids monomer Monosaccharides Nucleotide Function 1. Energy storage 2. Plant cellulose 1. Genetic material Lipids monomer Fatty Acids Function 1. Long term energy storage 2. Protective coverings 3. Insulation

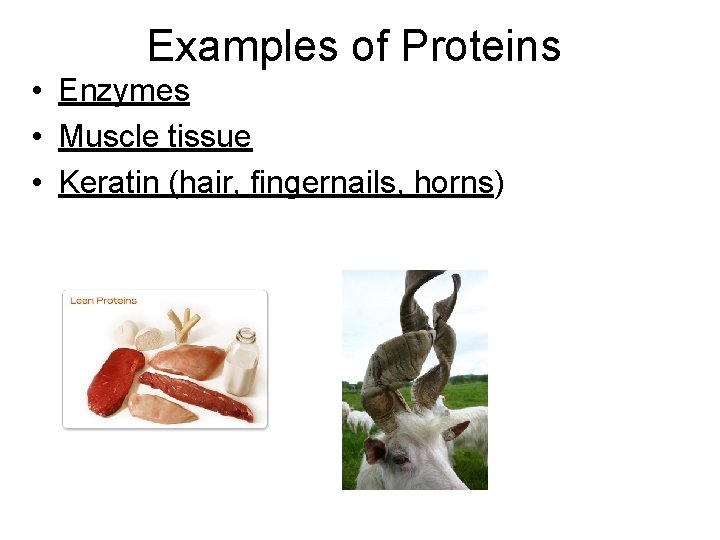
Examples of Proteins • Enzymes • Muscle tissue • Keratin (hair, fingernails, horns)

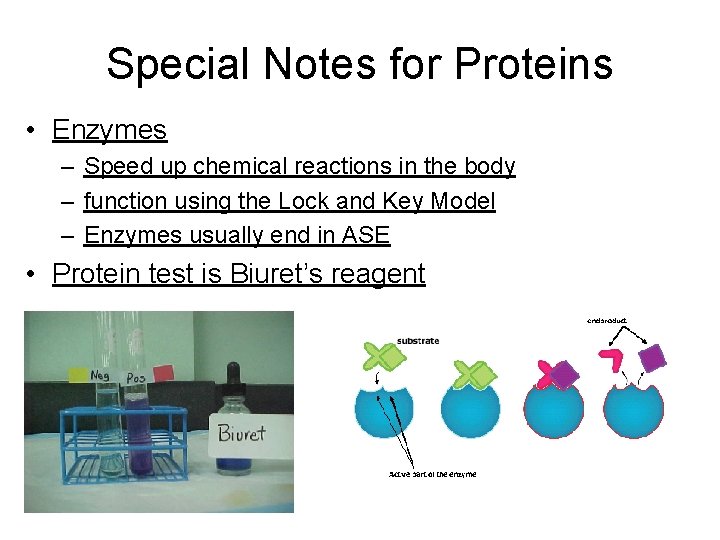
Special Notes for Proteins • Enzymes – Speed up chemical reactions in the body – function using the Lock and Key Model – Enzymes usually end in ASE • Protein test is Biuret’s reagent

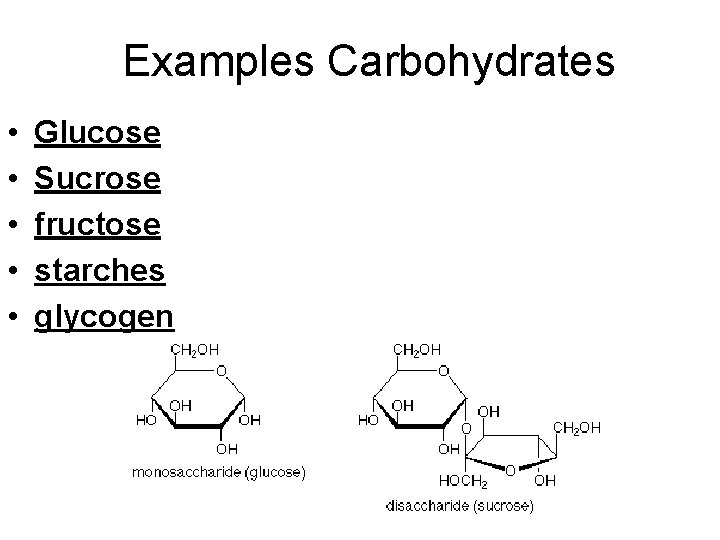
Examples Carbohydrates • • • Glucose Sucrose fructose starches glycogen

Special Notes for Carbohydrates • • Ratio of C: H: O is 1: 2: 1 (C 6 H 12 O 6) Starch Test is Iodine Sugar Test is Benedict’s Reagent Oftens ends in OSE

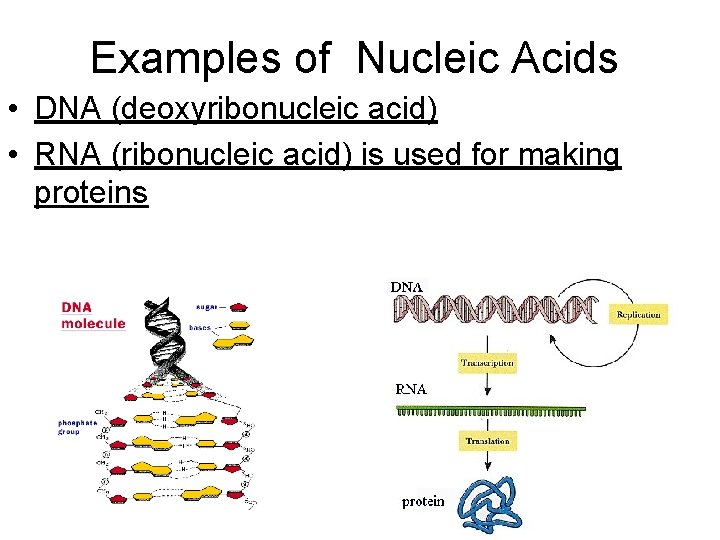
Examples of Nucleic Acids • DNA (deoxyribonucleic acid) • RNA (ribonucleic acid) is used for making proteins

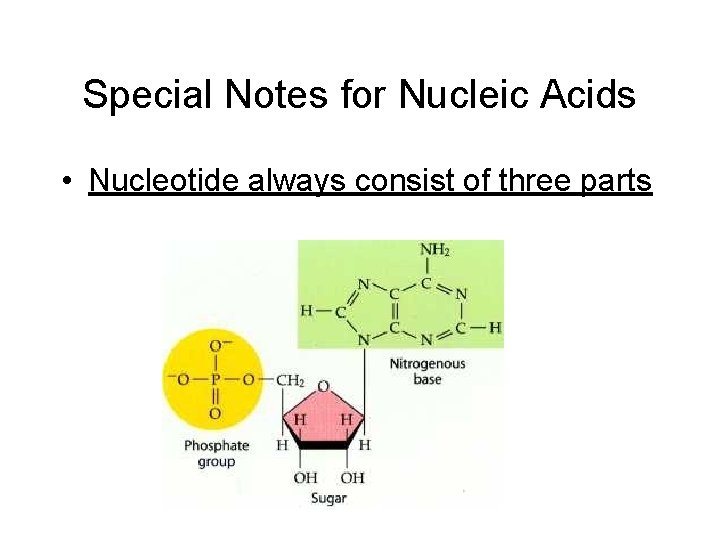
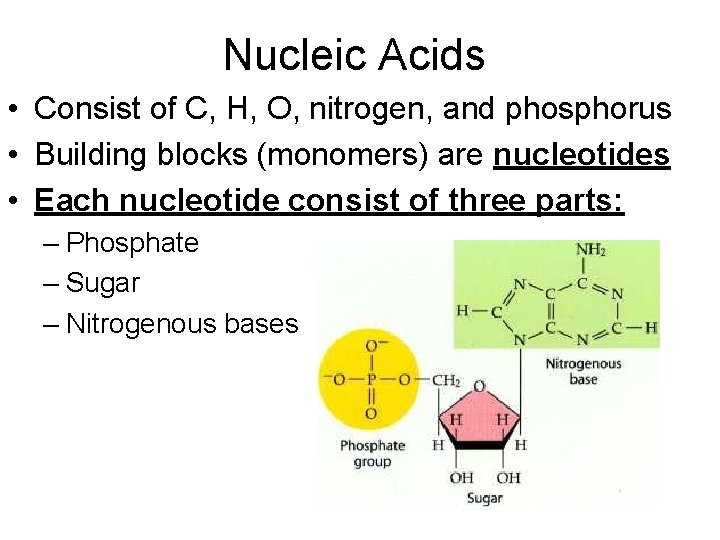
Special Notes for Nucleic Acids • Nucleotide always consist of three parts

Examples of Lipids • • Cell membrane Fats Oils Sterols – Cholesterol

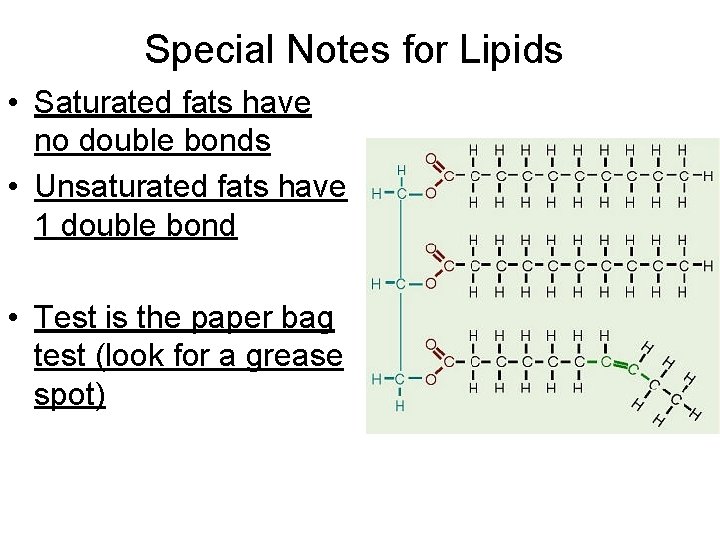
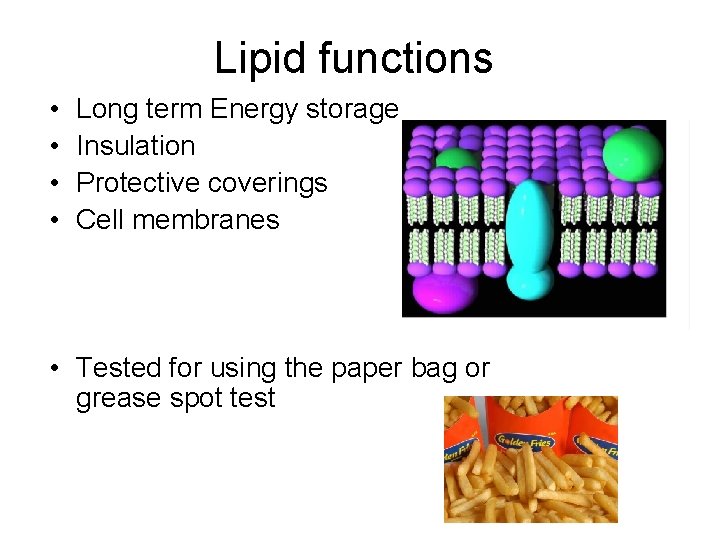
Special Notes for Lipids • Saturated fats have no double bonds • Unsaturated fats have 1 double bond • Test is the paper bag test (look for a grease spot)

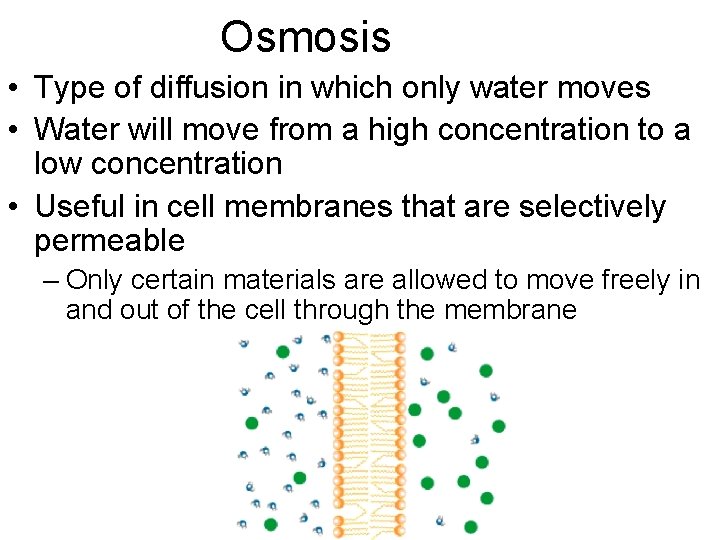
Osmosis • Type of diffusion in which only water moves • Water will move from a high concentration to a low concentration • Useful in cell membranes that are selectively permeable – Only certain materials are allowed to move freely in and out of the cell through the membrane

Nucleic Acids • Consist of C, H, O, nitrogen, and phosphorus • Building blocks (monomers) are nucleotides • Each nucleotide consist of three parts: – Phosphate – Sugar – Nitrogenous bases

Lipid functions • • Long term Energy storage Insulation Protective coverings Cell membranes • Tested for using the paper bag or grease spot test