Diffusion and Osmosis This powerpoints animations are best

Diffusion and Osmosis

This powerpoint’s animations are best viewed by full screen. Click on slideshow and play from start.
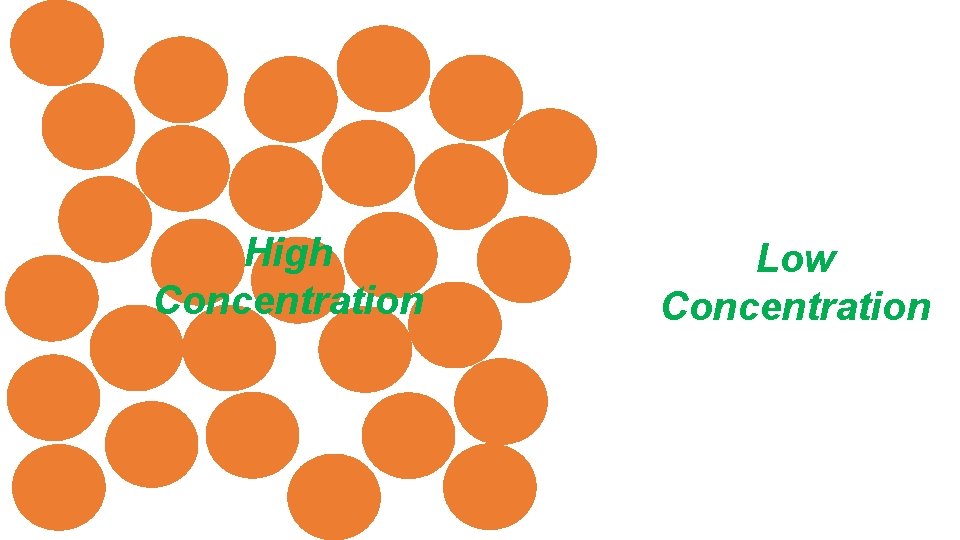
High Concentration Low Concentration

Equilibrium

Most Important Scientific Idea Ever! Big Idea in Science: High concentration Equilibrium Diffusion Low concentration Particles in a concentrated or high pressure area will want to move toward an area of lower concentration, or low pressure area. This movement toward less concentrated areas is called diffusion.

Example 1: Food colouring The food colouring is very concentrated. When you put it in water, it spreads out (diffuses) toward areas of lower concentration. High concentration went towards low concentration.

Example 2: Balloon The air pressure inside a balloon is much higher than the air pressure outside the balloon. This means that the particles inside the balloon are much more concentrated. If you puncture the balloon, the particles under high pressure will rush out toward the area of lower pressure. High pressure went towards low pressure.

Example 3: Body spray The contents of this can are under very high pressure. In other words, the concentration inside the can is very high. When it’s sprayed, the contents of the can rush towards areas of lower concentration. Because of this, you can smell the spray, even from far away. High concentration went towards low concentration.
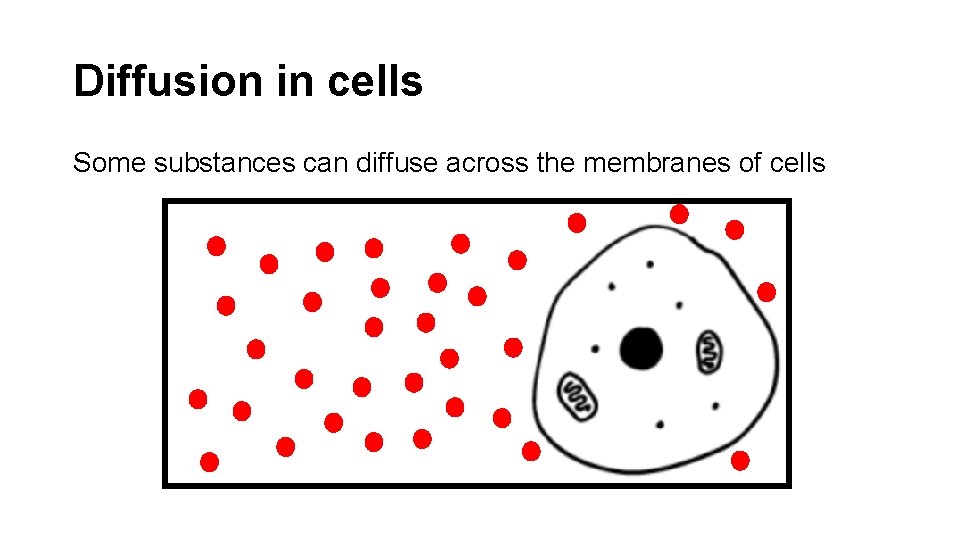
Diffusion in cells Some substances can diffuse across the membranes of cells
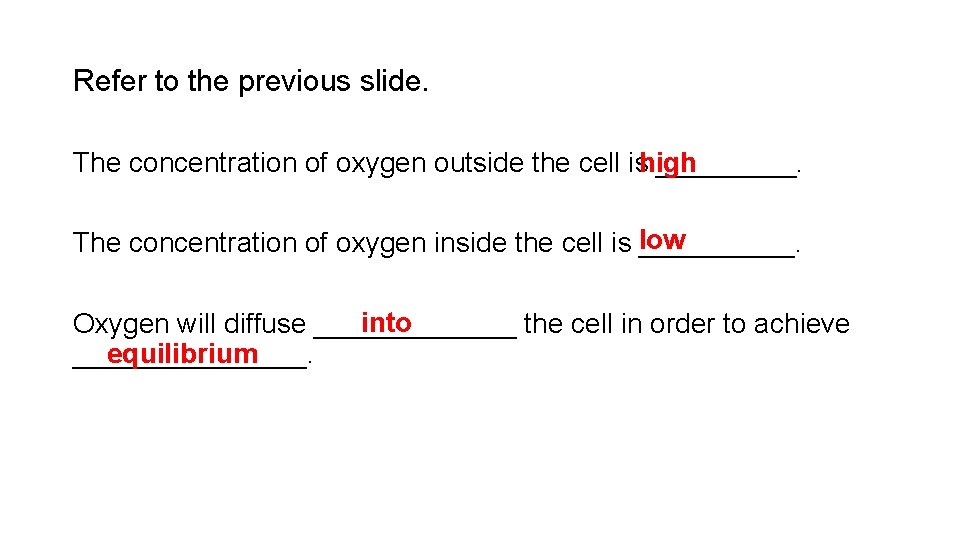
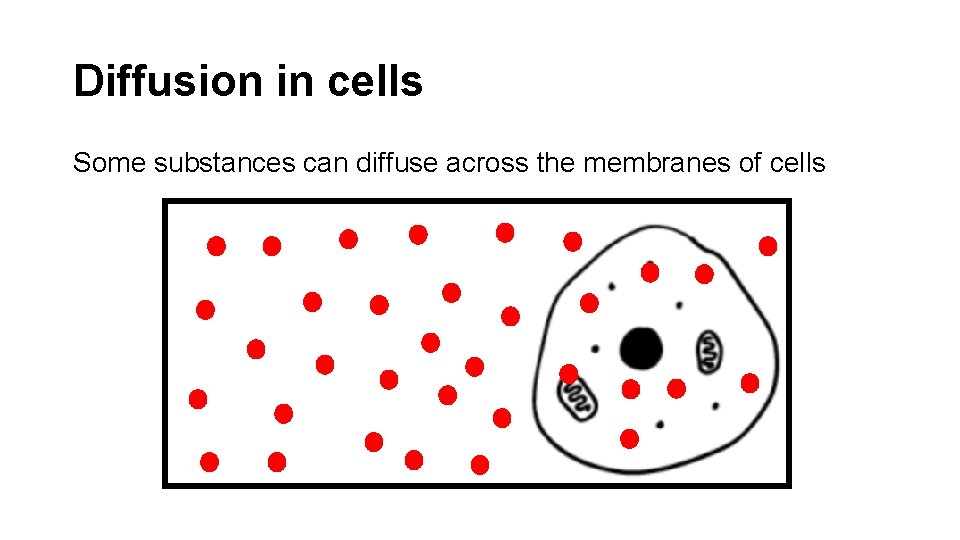
Refer to the previous slide. The concentration of oxygen outside the cell ishigh _____. The concentration of oxygen inside the cell is low _____. into Oxygen will diffuse _______ the cell in order to achieve equilibrium ________.
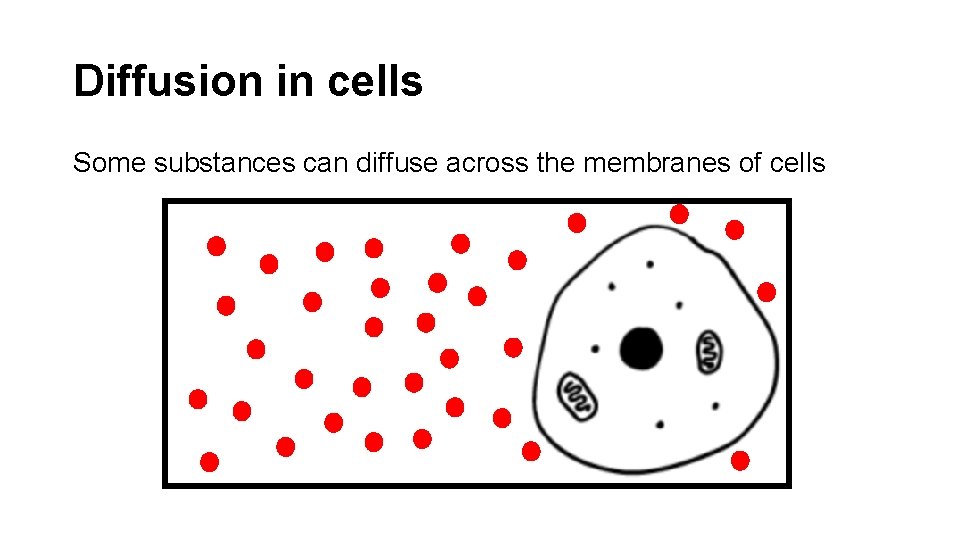
Diffusion in cells Some substances can diffuse across the membranes of cells
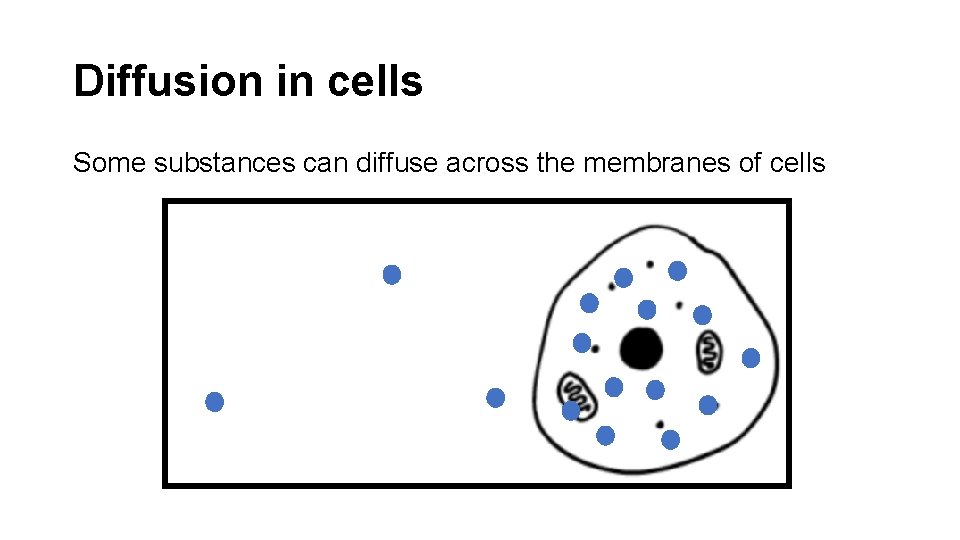
Diffusion in cells Some substances can diffuse across the membranes of cells
Diffusion in cells Some substances can diffuse across the membranes of cells
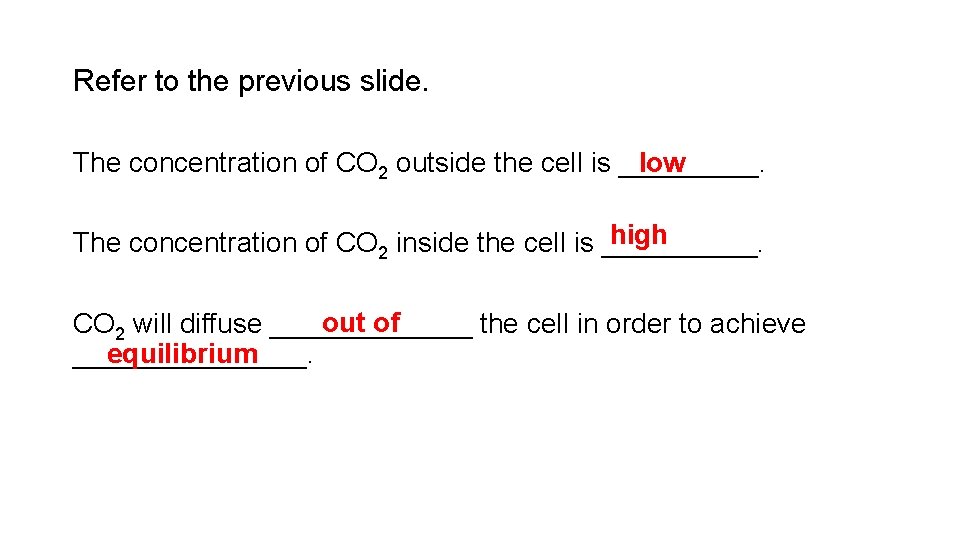
Refer to the previous slide. low The concentration of CO 2 outside the cell is _____. high The concentration of CO 2 inside the cell is _____. out of CO 2 will diffuse _______ the cell in order to achieve equilibrium ________.

Diffusion in cells Some substances can diffuse across the membranes of cells
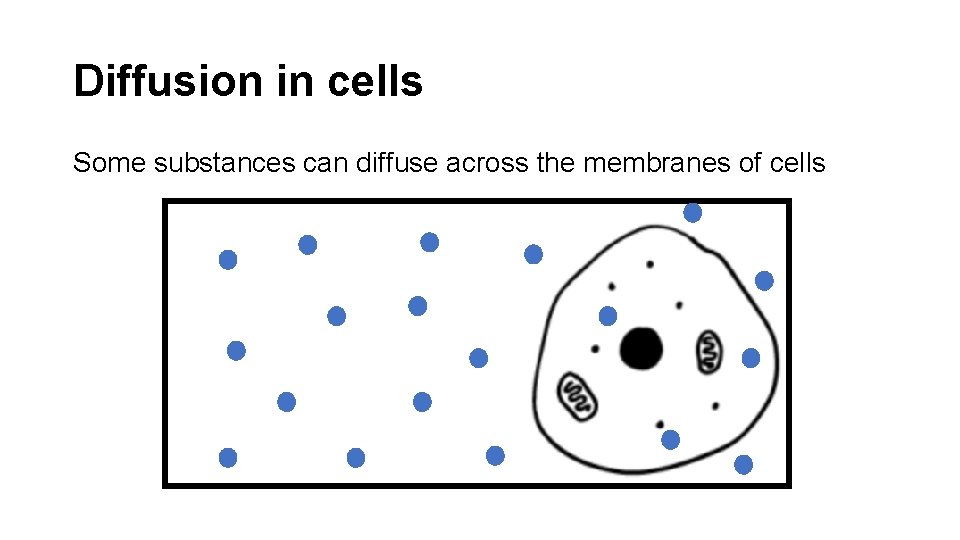
Diffusion in cells Some substances can diffuse across the membranes of cells
Let’s make Kool-Aid!

I dissolved Kool-Aid powder in water.
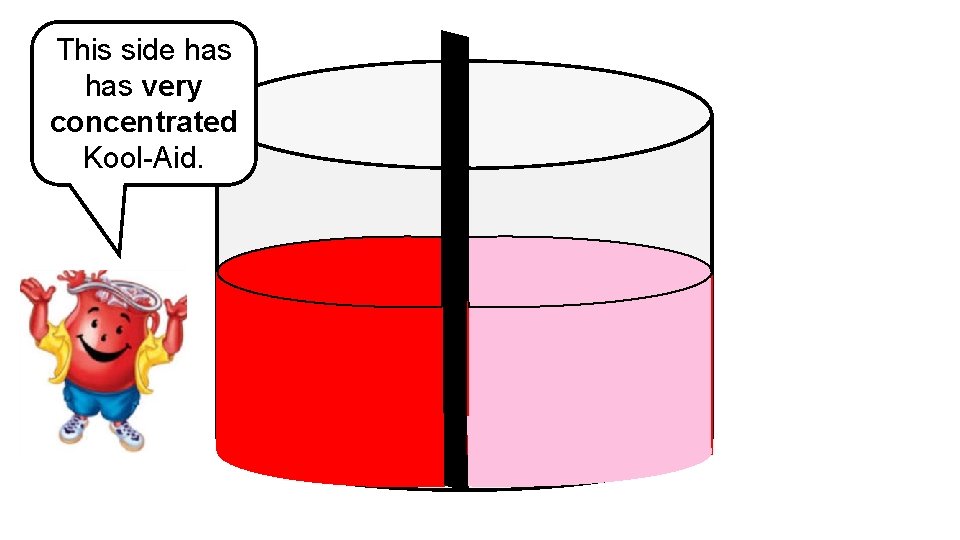
This side has very concentrated Kool-Aid.
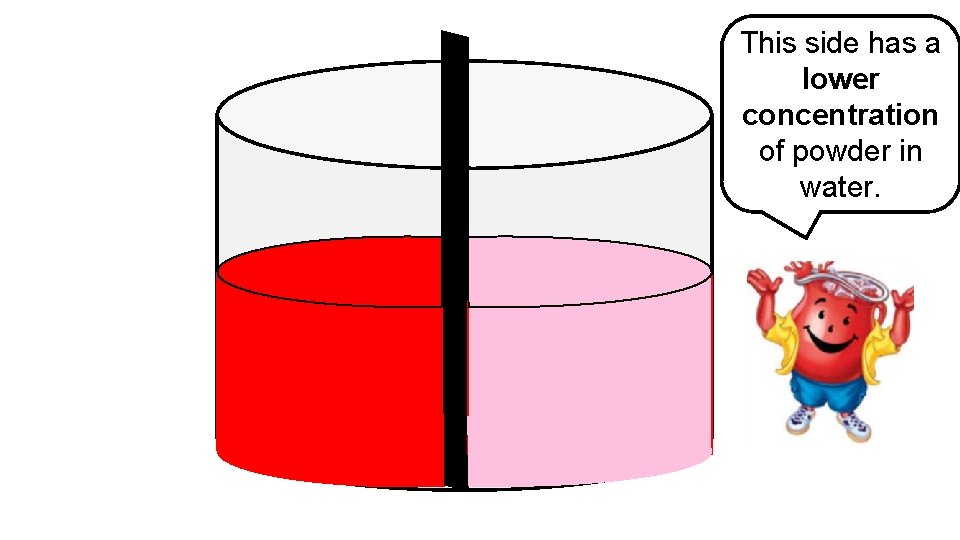
This side has a lower concentration of powder in water.
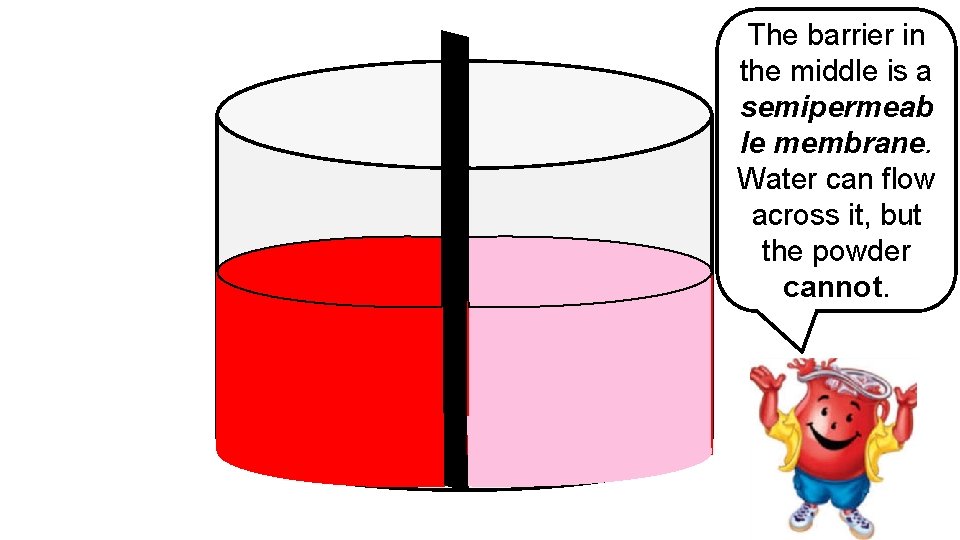
The barrier in the middle is a semipermeab le membrane. Water can flow across it, but the powder cannot.
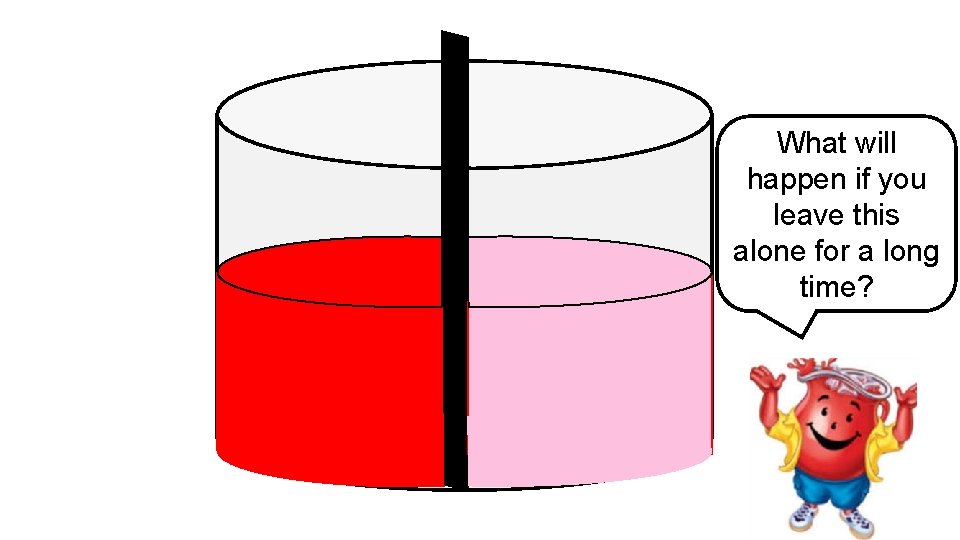
What will happen if you leave this alone for a long time?
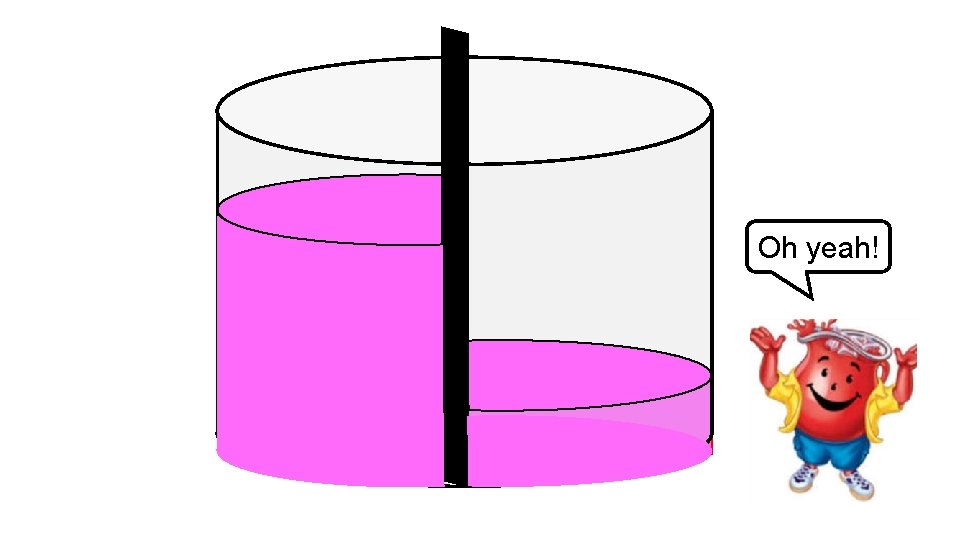
Oh yeah!
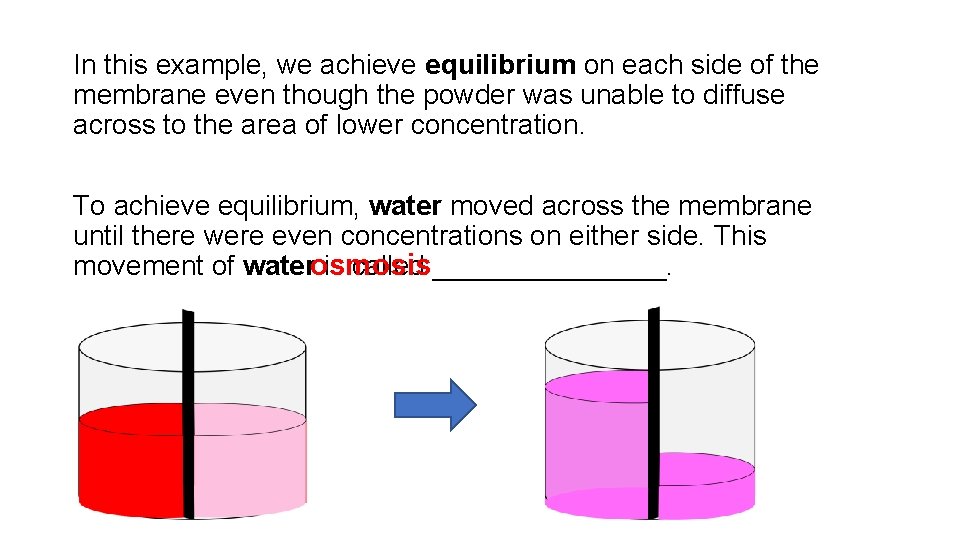
In this example, we achieve equilibrium on each side of the membrane even though the powder was unable to diffuse across to the area of lower concentration. To achieve equilibrium, water moved across the membrane until there were even concentrations on either side. This movement of waterosmosis is called ________.
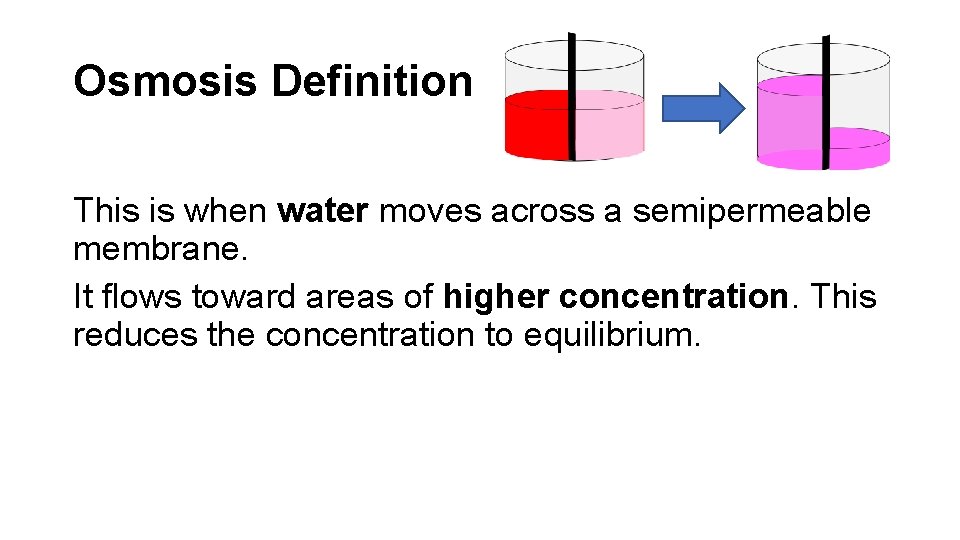
Osmosis Definition This is when water moves across a semipermeable membrane. It flows toward areas of higher concentration. This reduces the concentration to equilibrium.
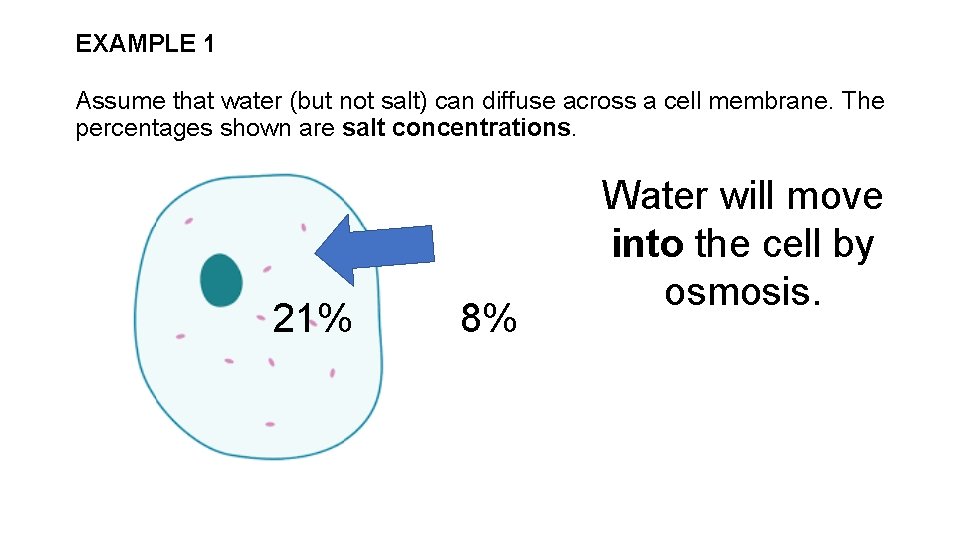
EXAMPLE 1 Assume that water (but not salt) can diffuse across a cell membrane. The percentages shown are salt concentrations. 21% 8% Water will move into the cell by osmosis.
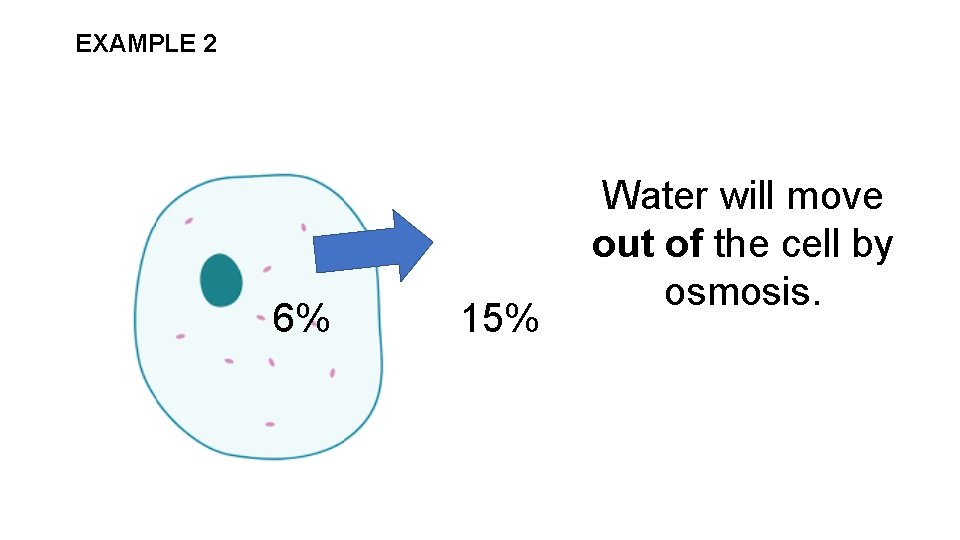
EXAMPLE 2 6% 15% Water will move out of the cell by osmosis.
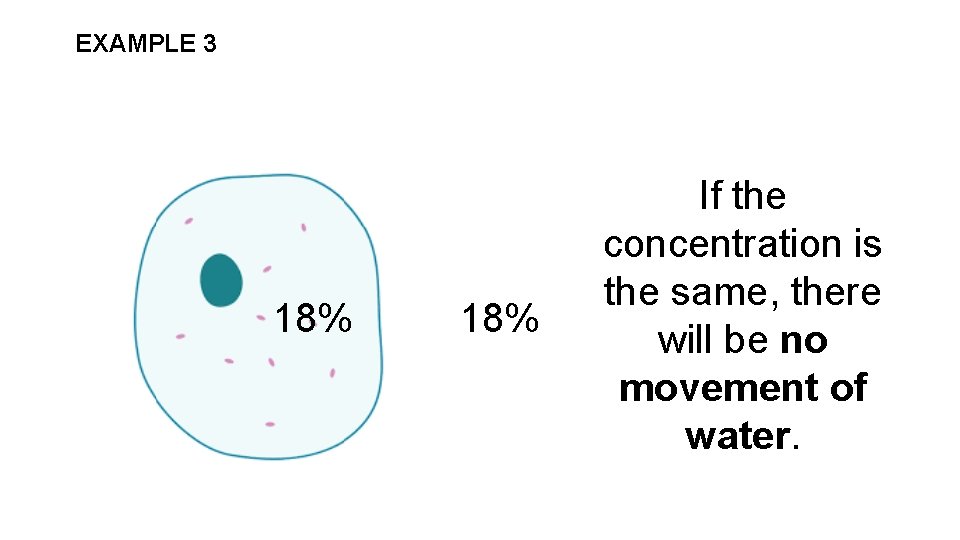
EXAMPLE 3 18% If the concentration is the same, there will be no movement of water.
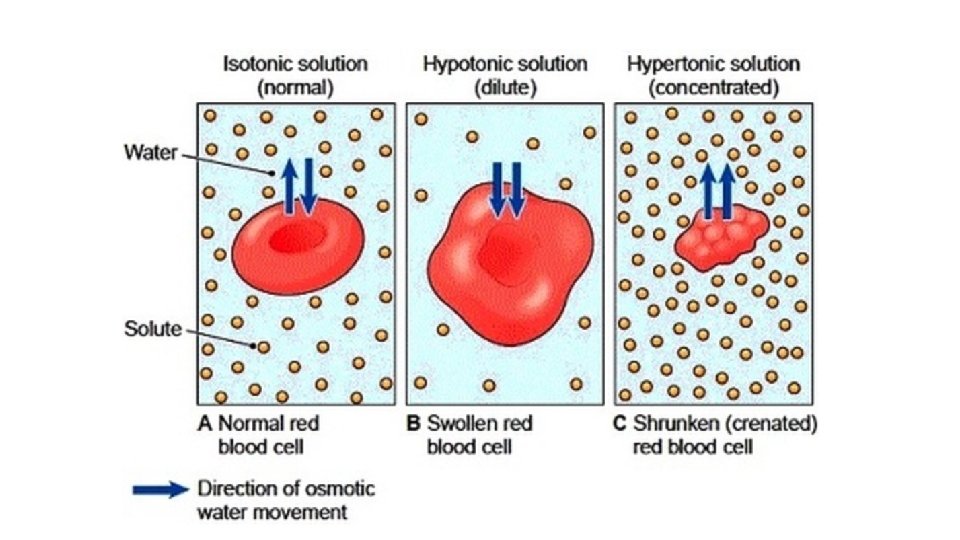

What happens when you eat lots of very salty food? Salty foods cause the concentration of salt inside your body to increase. To bring your salt concentration back toward equilibrium, water will move out of your cells by osmosis to reduce the salt concentration. This makes you thirsty. Water moved toward the area of higher concentration.

Why is giving somebody an intravenous solution of pure water a terrible idea? This is a way in which you can put medicine directly into someone’s blood stream. Your blood is naturally salty, so it’s very important to make sure the medicine’s salt concentration is the same. If you provide pure water, the salt concentration in your blood cells is higher. Therefore, water will move into your cells toward the area of higher concentration. This can cause your blood cells to swell and burst Water moved toward the area of higher concentration.

What happens if you put a fish from the ocean in a freshwater tank? Oceans are naturally salty, so the fish is used to living in an environment that has a high salt concentration. The fish’s cells have a high salt concentration as well to survive in this water. If you bring this fish home and put it in a freshwater tank, the water will move into the fish’s cells, toward the area of high salt concentration. This can cause the fish’s cells to swell and burst. Water moved toward the area of higher concentration.
- Slides: 32