Diffusion and Osmosis Outline Learn the concepts of

![Effect of Water on Cells • Hypertonic Environment – High [solute], low [water] • Effect of Water on Cells • Hypertonic Environment – High [solute], low [water] •](https://slidetodoc.com/presentation_image_h/7a841bf1757855090e58fb4bb1fa9980/image-10.jpg)





- Slides: 20

Diffusion and Osmosis

Outline • Learn the concepts of: • Explore diffusion in a colloid • – – Relationship between diffusion & size Explore diffusion & osmosis in a dialysis bag – – – • Diffusion, osmosis, semi-permeable membrane, isotonic, hypertonic, & hypotonic Dialysis bag experiment Semi-permeable membrane Water, glucose, starch Plant/animal cells exposure to water – – – Hypertonic Env. Hypotonic Env. Isotonic Env. 1 2 3 4

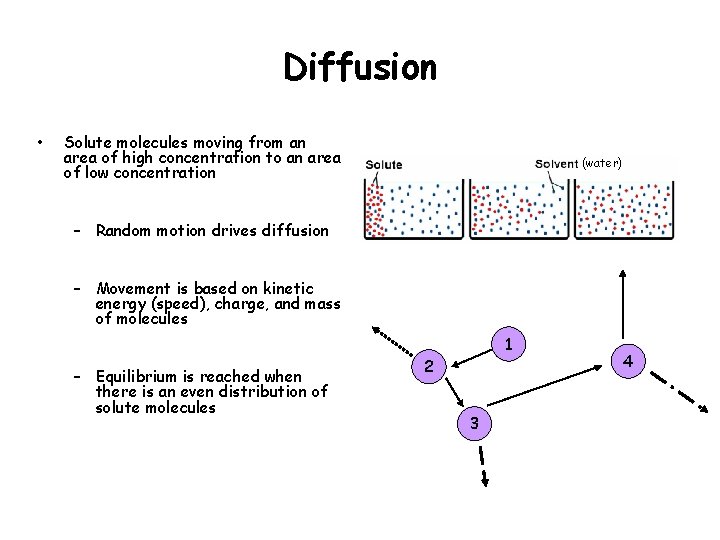
Diffusion • Solute molecules moving from an area of high concentration to an area of low concentration (water) – Random motion drives diffusion – Movement is based on kinetic energy (speed), charge, and mass of molecules 1 – Equilibrium is reached when there is an even distribution of solute molecules 2 3 4

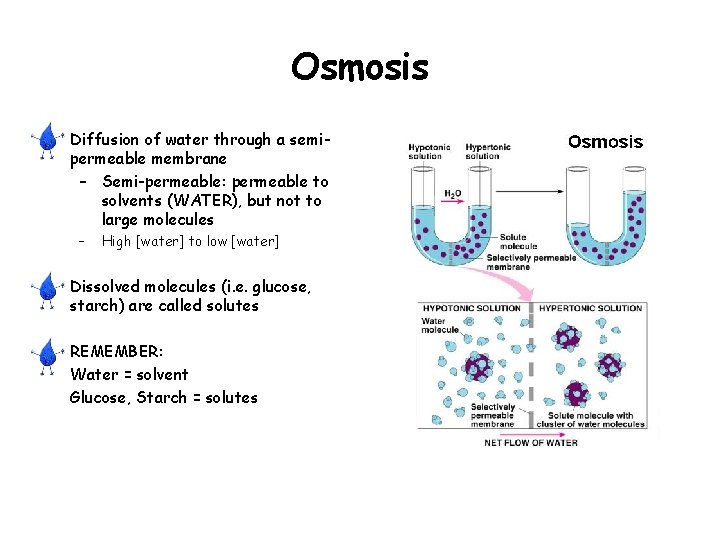
Osmosis • Diffusion of water through a semipermeable membrane – Semi-permeable: permeable to solvents (WATER), but not to large molecules – High [water] to low [water] • Dissolved molecules (i. e. glucose, starch) are called solutes • REMEMBER: Water = solvent Glucose, Starch = solutes

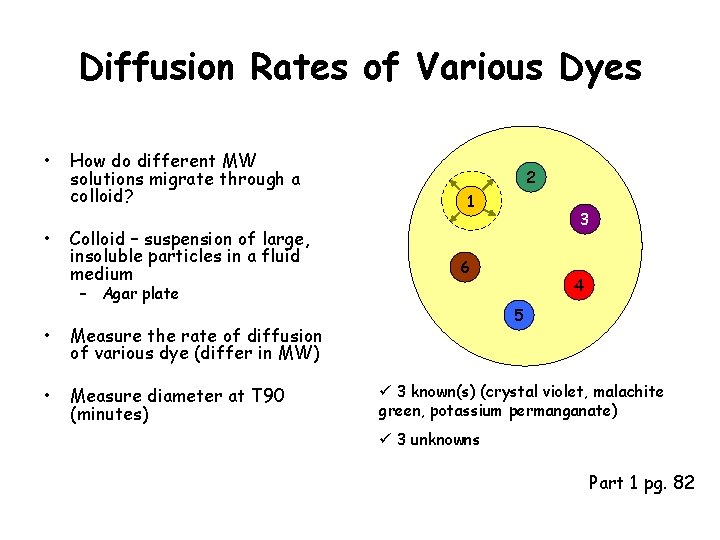
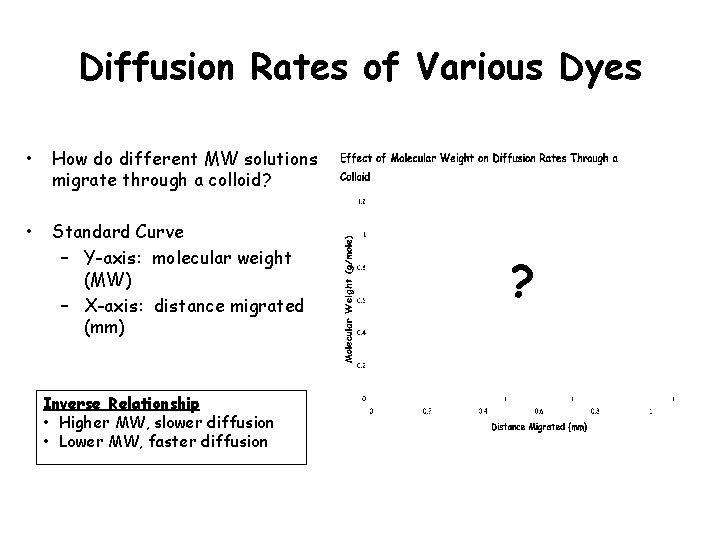
Diffusion Rates of Various Dyes • • How do different MW solutions migrate through a colloid? Colloid – suspension of large, insoluble particles in a fluid medium 2 1 6 – Agar plate • Measure the rate of diffusion of various dye (differ in MW) • Measure diameter at T 90 (minutes) 3 4 5 ü 3 known(s) (crystal violet, malachite green, potassium permanganate) ü 3 unknowns Part 1 pg. 82

Diffusion Rates of Various Dyes • How do different MW solutions migrate through a colloid? • Standard Curve – Y-axis: molecular weight (MW) – X-axis: distance migrated (mm) Inverse Relationship • Higher MW, slower diffusion • Lower MW, faster diffusion ?

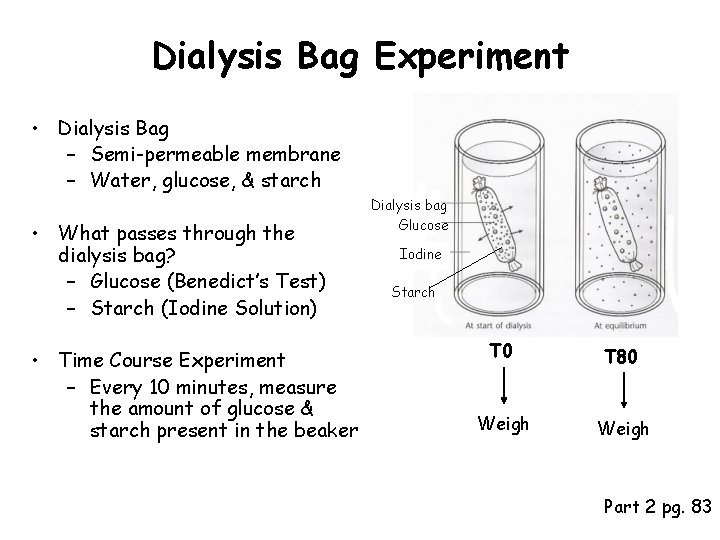
Dialysis Bag Experiment • Dialysis Bag – Semi-permeable membrane – Water, glucose, & starch • What passes through the dialysis bag? – Glucose (Benedict’s Test) – Starch (Iodine Solution) • Time Course Experiment – Every 10 minutes, measure the amount of glucose & starch present in the beaker Dialysis bag Glucose Iodine Starch T 0 T 80 Weigh Part 2 pg. 83

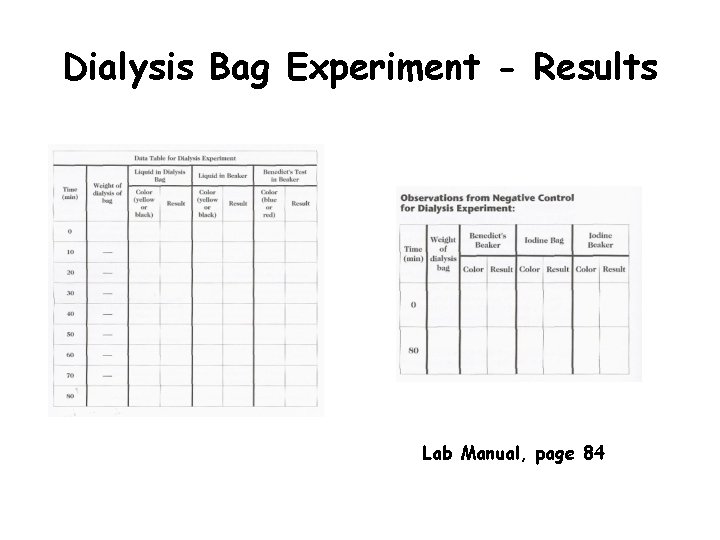
Dialysis Bag Experiment - Results Lab Manual, page 84

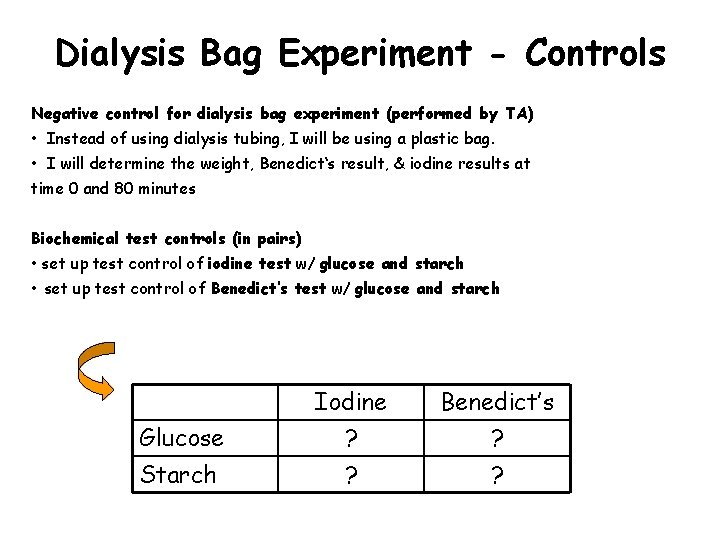
Dialysis Bag Experiment - Controls Negative control for dialysis bag experiment (performed by TA) • Instead of using dialysis tubing, I will be using a plastic bag. • I will determine the weight, Benedict‘s result, & iodine results at time 0 and 80 minutes Biochemical test controls (in pairs) • set up test control of iodine test w/ glucose and starch • set up test control of Benedict‘s test w/ glucose and starch Glucose Iodine ? Benedict’s ? Starch ? ?
![Effect of Water on Cells Hypertonic Environment High solute low water Effect of Water on Cells • Hypertonic Environment – High [solute], low [water] •](https://slidetodoc.com/presentation_image_h/7a841bf1757855090e58fb4bb1fa9980/image-10.jpg)
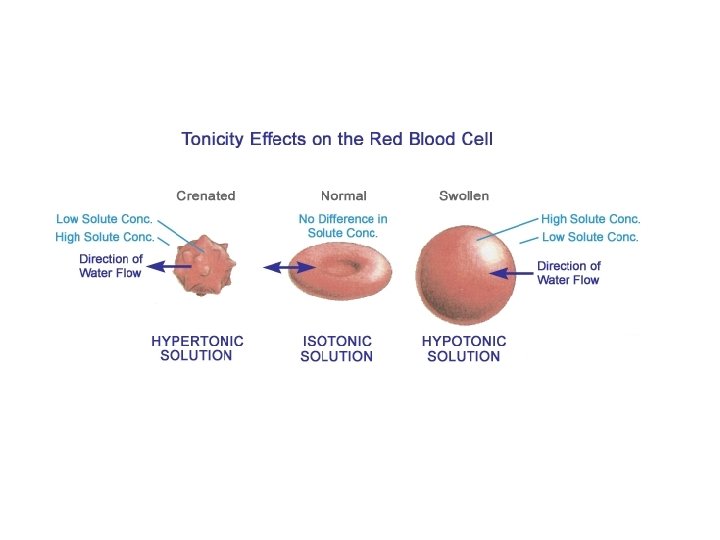
Effect of Water on Cells • Hypertonic Environment – High [solute], low [water] • Hypotonic Environment – High [water], low [solute] Hypertonic • Isotonic Environment – [water] = [solute] Hypotonic Isotonic Part 3 pg. 85

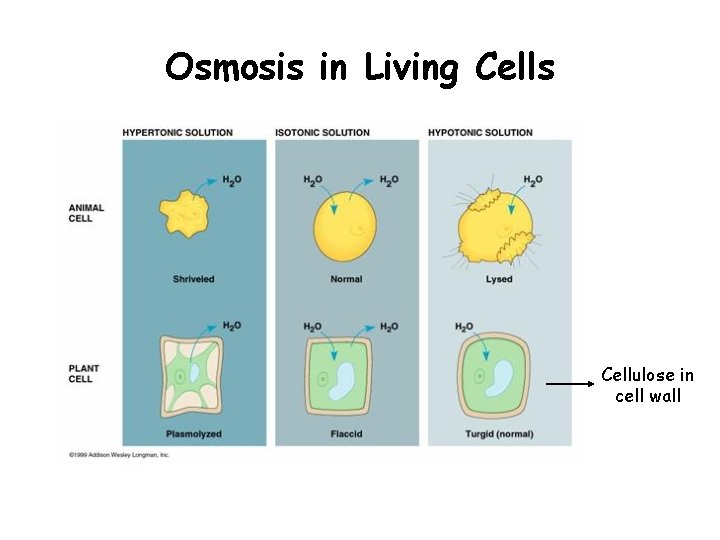
Osmosis in Living Cells Cellulose in cell wall

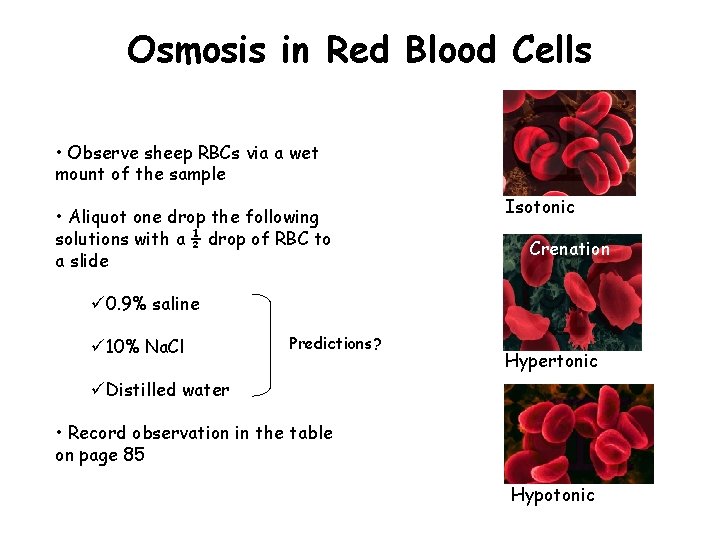
Osmosis in Red Blood Cells • Observe sheep RBCs via a wet mount of the sample • Aliquot one drop the following solutions with a ½ drop of RBC to a slide Isotonic Crenation ü 0. 9% saline ü 10% Na. Cl Predictions? Hypertonic üDistilled water • Record observation in the table on page 85 Hypotonic

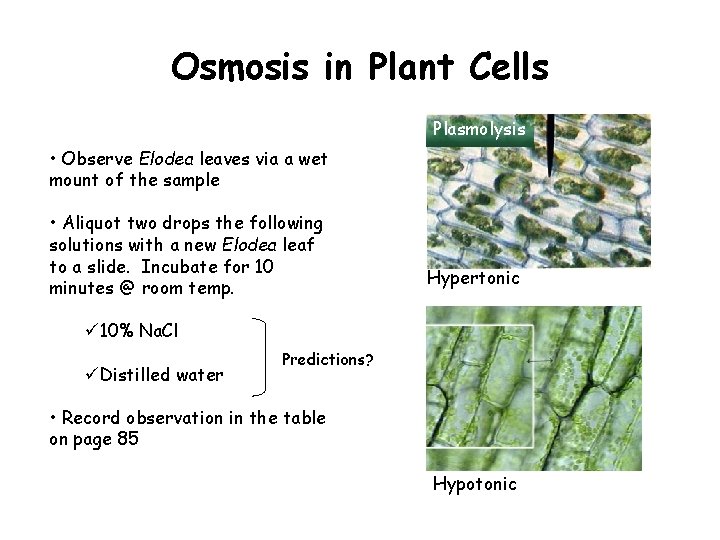
Osmosis in Plant Cells Plasmolysis • Observe Elodea leaves via a wet mount of the sample • Aliquot two drops the following solutions with a new Elodea leaf to a slide. Incubate for 10 minutes @ room temp. Hypertonic ü 10% Na. Cl üDistilled water Predictions? • Record observation in the table on page 85 Hypotonic

Plan of attack. . . ü Set-up Part I – Molecular Weight Diffusion in Colloid ü ü Set up Part II – Dialysis Bag ü ü Incubate for 90 minutes Time course: 80 minutes (time-point every 10 minutes) Part III – Osmosis in Living Cells

Animations Dialysis Bag Experiment • http: //ccollege. hccs. cc. tx. us/instru/Biology/All. Study Pages/Diffusion_Osmosis/Baggif. swf Elodea Cell • http: //ccollege. hccs. cc. tx. us/instru/Biology/All. Study Pages/Diffusion_Osmosis/Elodeagif. swf Osmosis • http: //ull. chemistry. uakron. edu/genobc/animations/os mosis. mov

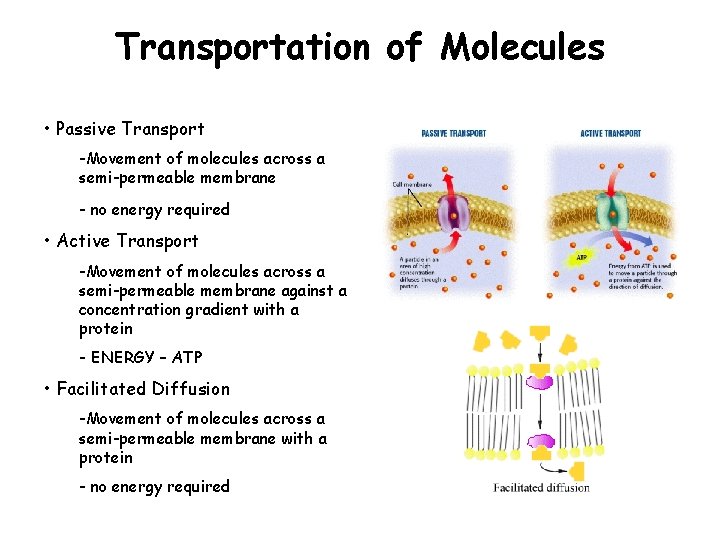
Transportation of Molecules • Passive Transport -Movement of molecules across a semi-permeable membrane - no energy required • Active Transport -Movement of molecules across a semi-permeable membrane against a concentration gradient with a protein - ENERGY – ATP • Facilitated Diffusion -Movement of molecules across a semi-permeable membrane with a protein - no energy required

Effect of Water on RBC


