Diffuse Parenchymal Lung Disease Diagnostic and Therapeutic Challenges





















- Slides: 52

Diffuse Parenchymal Lung Disease Diagnostic and Therapeutic Challenges Ali Bin Sarwar Zubairi; MBBS, FCCP

Disclosures • I have no financial disclosures related to this presentation • There will be no discussion of the off-label use of therapeutics

Outline • Introduction • Diagnostic criteria and challenges • Treatment options and challenges • Conclusion

Diffuse Parenchymal Lung Disease (DPLD) • Diverse group of disorders • Over 100 diseases can result in interstitial involvement • Classified together because of similar — clinical, — physiologic — plain radiographic manifestations

Initial Description • In 1892, Osler described chronic interstitial pneumonia as ‘cirrhosis of the lung’ Osler W. The Principles and Practice of Medicine New York, NY: D. Appleton & Co; 1892 • In 1944, Hamman and Rich described 4 cases of acute interstitial fibrosis Hamman L, Rich A. Acute diffuse interstitial lung fibrosis Bull Johns Hopkins Hosp. 1944; 74: 177 -212

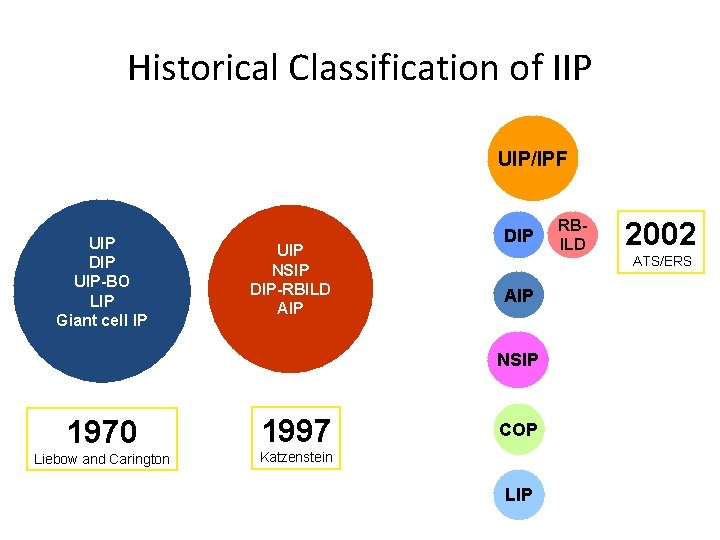
Historical Classification of IIP UIP/IPF UIP DIP UIP-BO LIP Giant cell IP UIP NSIP DIP-RBILD AIP DIP AIP NSIP 1970 1997 Liebow and Carington Katzenstein COP LIP RBILD 2002 ATS/ERS

Statements Am J Respir Crit Care Med Vol 165, pp 277 -304, 2002 Am J Respir Crit Care Med Vol 188, Iss. 6, pp 733– 748, Sep 2013

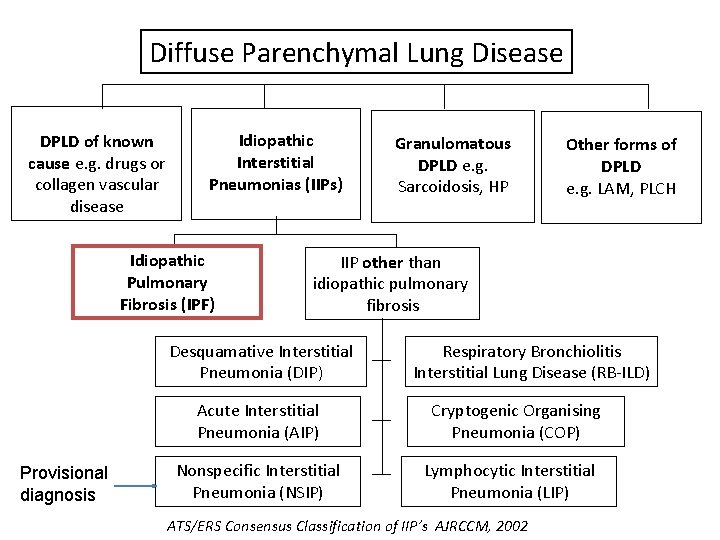
Diffuse Parenchymal Lung Disease DPLD of known cause e. g. drugs or collagen vascular disease Idiopathic Interstitial Pneumonias (IIPs) Idiopathic Pulmonary Fibrosis (IPF) Other forms of DPLD e. g. LAM, PLCH IIP other than idiopathic pulmonary fibrosis Desquamative Interstitial Pneumonia (DIP) Acute Interstitial Pneumonia (AIP) Provisional diagnosis Granulomatous DPLD e. g. Sarcoidosis, HP Nonspecific Interstitial Pneumonia (NSIP) Respiratory Bronchiolitis Interstitial Lung Disease (RB-ILD) Cryptogenic Organising Pneumonia (COP) Lymphocytic Interstitial Pneumonia (LIP) ATS/ERS Consensus Classification of IIP’s AJRCCM, 2002

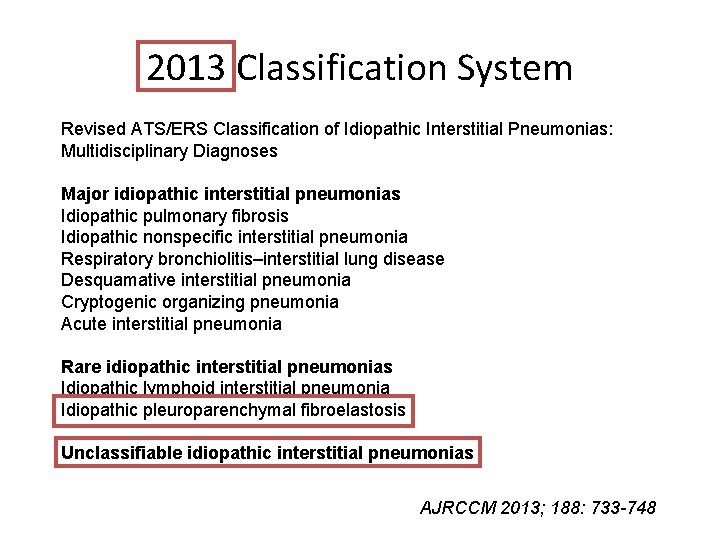
2013 Classification System Revised ATS/ERS Classification of Idiopathic Interstitial Pneumonias: Multidisciplinary Diagnoses Major idiopathic interstitial pneumonias Idiopathic pulmonary fibrosis Idiopathic nonspecific interstitial pneumonia Respiratory bronchiolitis–interstitial lung disease Desquamative interstitial pneumonia Cryptogenic organizing pneumonia Acute interstitial pneumonia Rare idiopathic interstitial pneumonias Idiopathic lymphoid interstitial pneumonia Idiopathic pleuroparenchymal fibroelastosis Unclassifiable idiopathic interstitial pneumonias AJRCCM 2013; 188: 733 -748

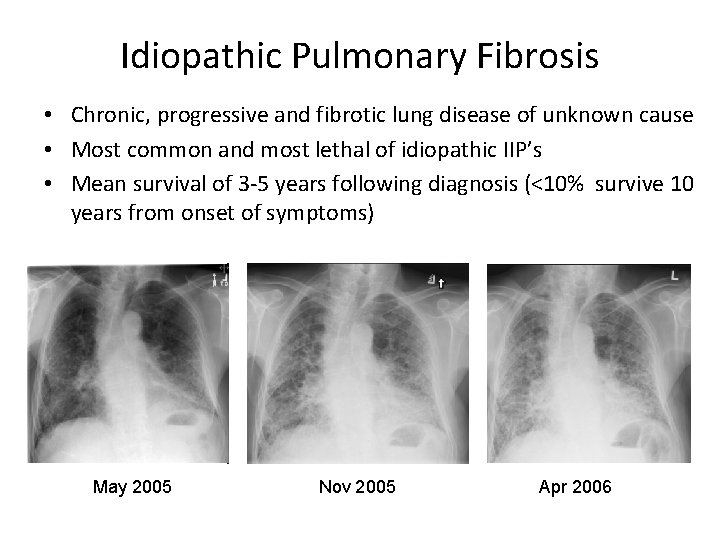
Idiopathic Pulmonary Fibrosis • Chronic, progressive and fibrotic lung disease of unknown cause • Most common and most lethal of idiopathic IIP’s • Mean survival of 3 -5 years following diagnosis (<10% survive 10 years from onset of symptoms) May 2005 Nov 2005 Apr 2006

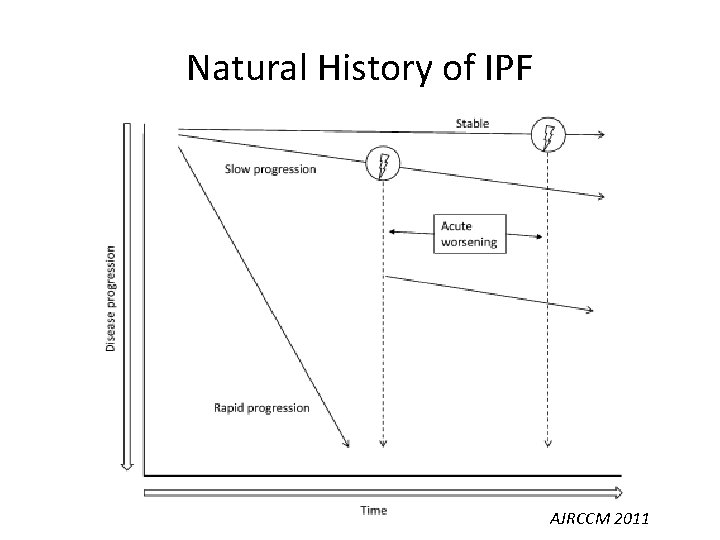
Natural History of IPF AJRCCM 2011

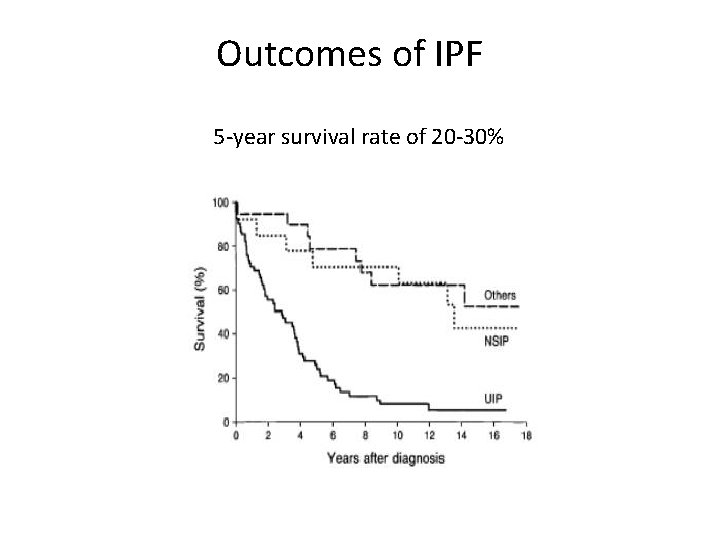
Outcomes of IPF 5 -year survival rate of 20 -30%

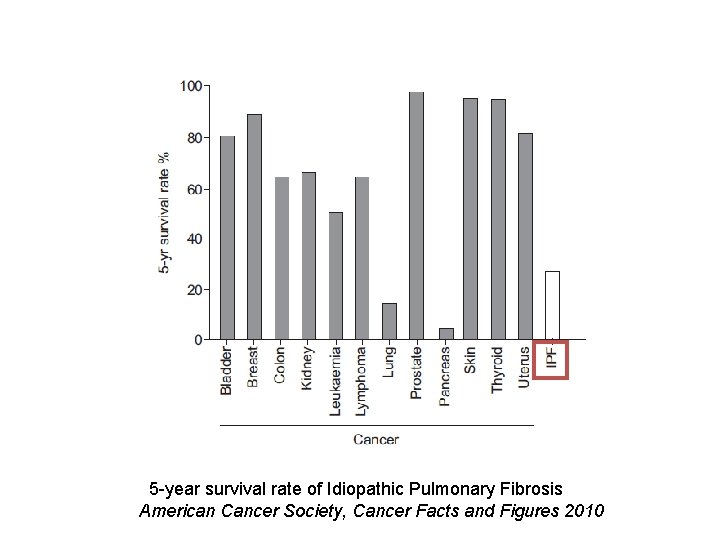
5 -year survival rate of Idiopathic Pulmonary Fibrosis American Cancer Society, Cancer Facts and Figures 2010

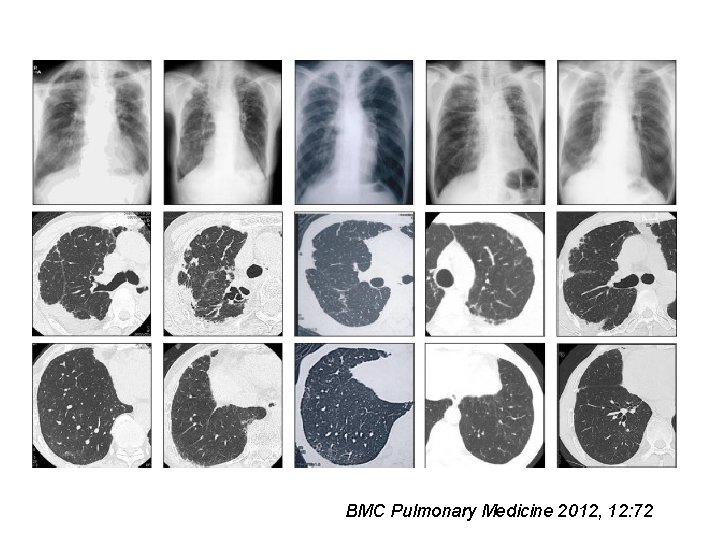
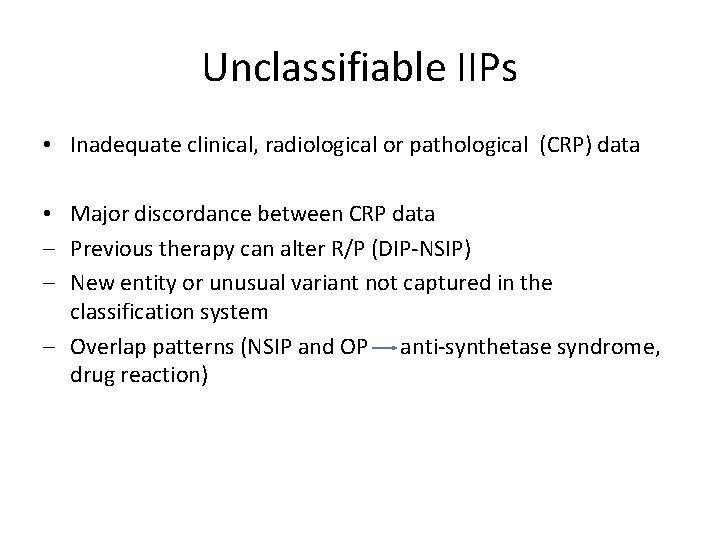
Idiopathic Pleuroparenchymal Fibroelastosis (IPPF) Median age = 57 years M=F PTX common Upper lobe-predominant pulmonary fibrosis (Idiopathic upper lobe fibrosis/IPUF)- Japanese literature • Progressive disease in 70% • Death in 50% (4 months-2 years) • • Frankel SK et al. CHEST 2004; 126: 2007 Reddy TL et al. Eur Respir J 2012; 40: 377 -385

BMC Pulmonary Medicine 2012, 12: 72

Unclassifiable IIPs • Inadequate clinical, radiological or pathological (CRP) data • Major discordance between CRP data ‒ Previous therapy can alter R/P (DIP-NSIP) ‒ New entity or unusual variant not captured in the classification system ‒ Overlap patterns (NSIP and OP anti-synthetase syndrome, drug reaction)

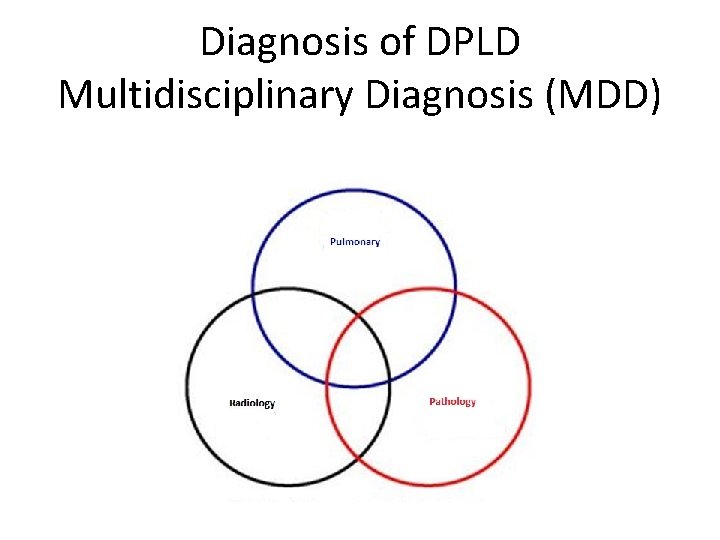
Diagnosis of DPLD Multidisciplinary Diagnosis (MDD)

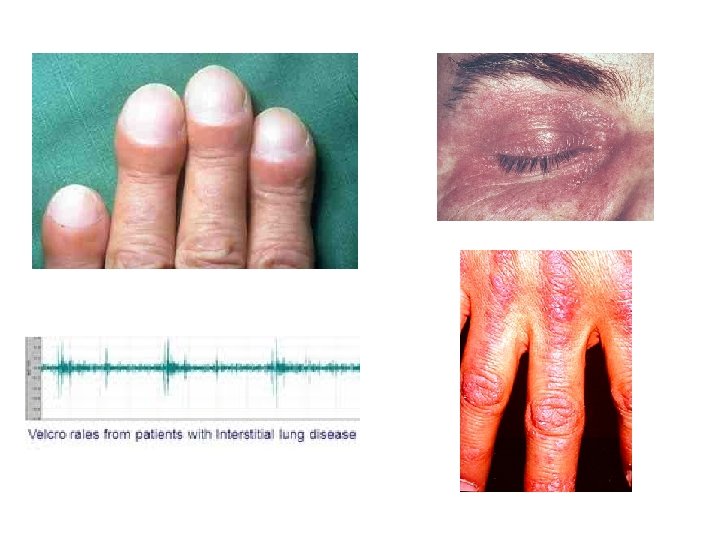
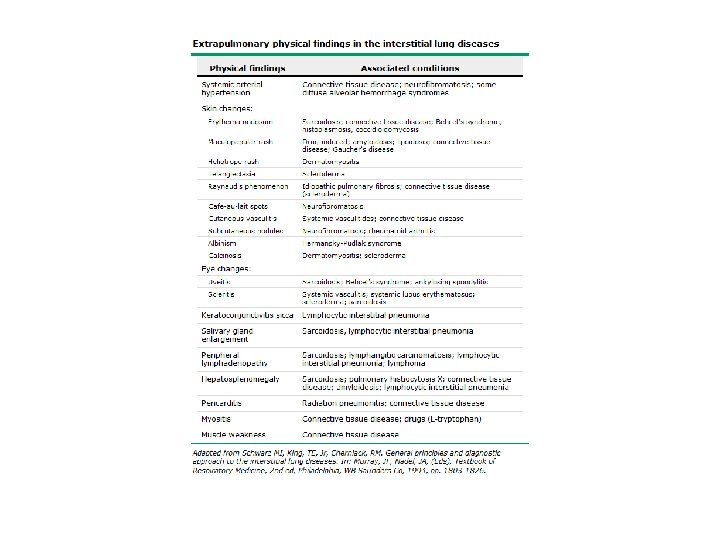
Diagnostic Challenges • Delay in diagnosis − non-specific history and physical − symptoms attributed to aging or smoking • Quality of images • Expertise to obtain surgical lung biopsy • Experience − to interpret HRCT scans − to read histopathology


Smoking-related ILD’s • DIP, RB-ILD • PLCH • IPF Chest /124 /4/October, 2003

pneumotox. com



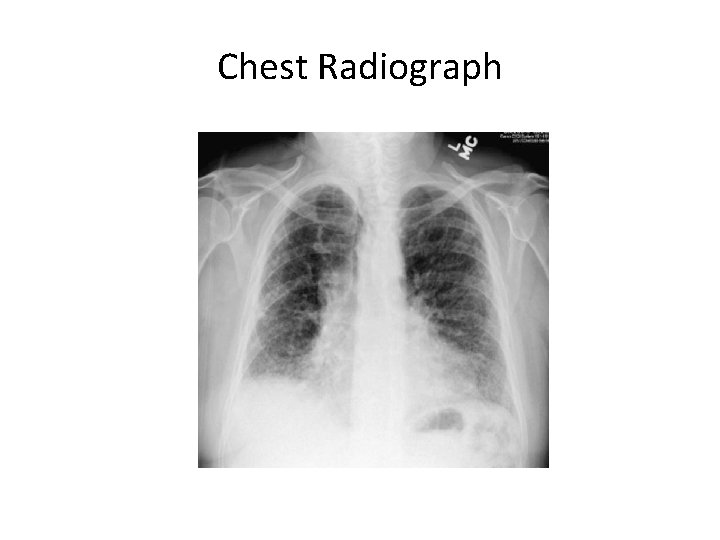
Chest Radiograph

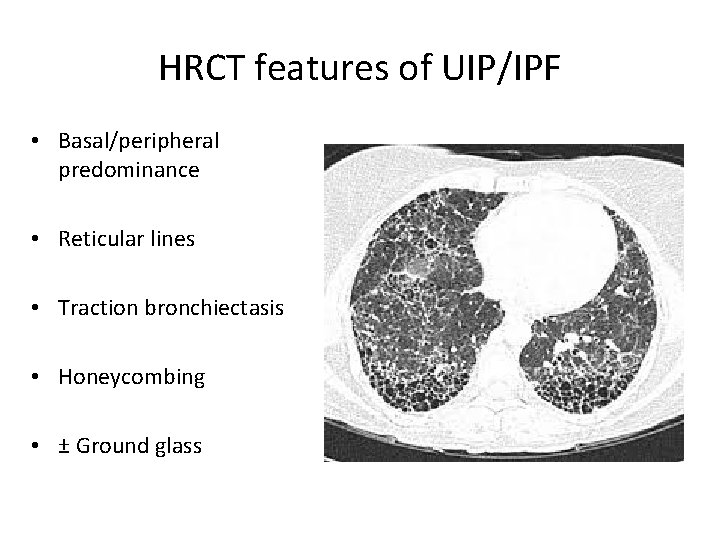
HRCT features of UIP/IPF • Basal/peripheral predominance • Reticular lines • Traction bronchiectasis • Honeycombing • ± Ground glass



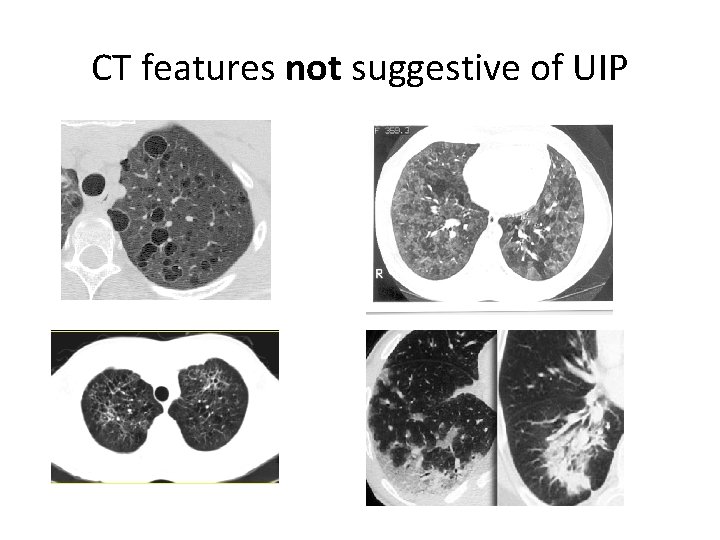
CT features not suggestive of UIP


Treatment Options 1. Drugs 2. Adjunctive therapy: Domiciliary oxygen Pulmonary rehabilitation 3. Lung transplantation

Therapeutic Challenges • − − − Lack of current treatment options No survival benefit Limited clinical trials Non-availability/quality of drugs • Non-availability of lung transplantation • Economic burden

Corticosteroids • Chronic inflammation- once thought to be a key factor • No RCT’s • Response rate of 0 -17% • No survival benefit • Plethora of side effects Douglas WW. AJRCCM

Azathioprine and Cyclophsphamide • ATS/ERS 2000 ₋ 6 months of either oral AZA (2 -3 mg/K/day) or oral CYC (2 mg/Kg/day) + Prednisone (0. 5 mg/Kg/day) for 4 weeks with subsequent taper ₋ Lack of survival benefit and serious side effects • ATS/ERS/JRS/ALAT 2011 ₋ No proven benefit • Survey of Pulmonologists showed that 50% used a regimen of either 2 or 3 drugs Peikert T Respir Med 2008

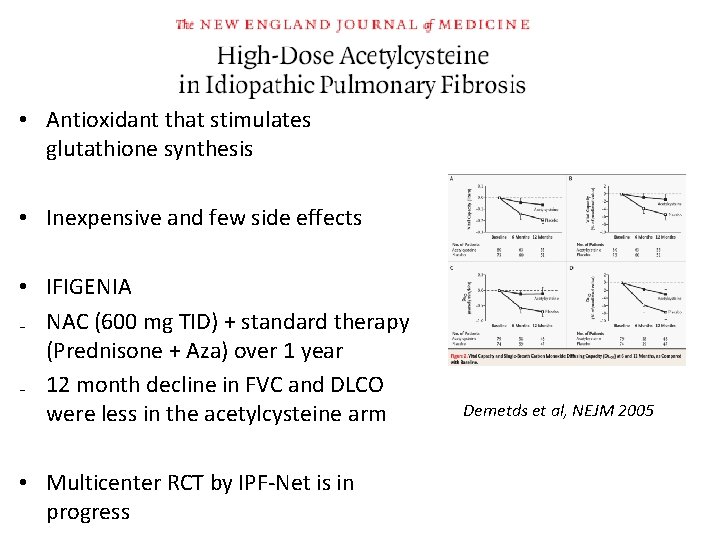
• Antioxidant that stimulates glutathione synthesis • Inexpensive and few side effects • IFIGENIA ₋ NAC (600 mg TID) + standard therapy (Prednisone + Aza) over 1 year ₋ 12 month decline in FVC and DLCO were less in the acetylcysteine arm • Multicenter RCT by IPF-Net is in progress Demetds et al, NEJM 2005

• First study that compared the effectiveness of combined treatment to placebo • 3 -arm multicenter clinical trial sponsored by NHLBI • Randomized to receive triple drug regimen, NAC alone or placebo for 60 weeks (1: 1: 1) PANTHER-IPF NEJM 2012

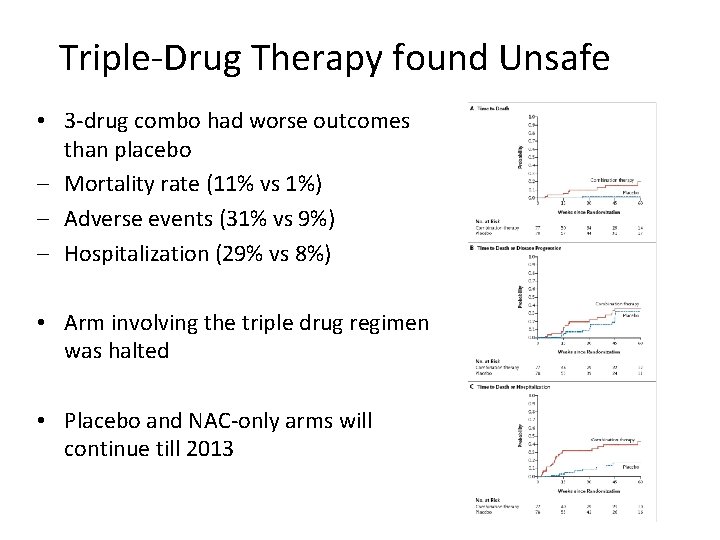
Triple-Drug Therapy found Unsafe • 3 -drug combo had worse outcomes than placebo ‒ Mortality rate (11% vs 1%) ‒ Adverse events (31% vs 9%) ‒ Hospitalization (29% vs 8%) • Arm involving the triple drug regimen was halted • Placebo and NAC-only arms will continue till 2013

Search for new therapies • • • IFN- -Ib Cyclosporin-A Colcochine Bosentan Etanercept Imatanib Sildenafil Anticoagulants Mycophenolate Pirfenidone Trial failures

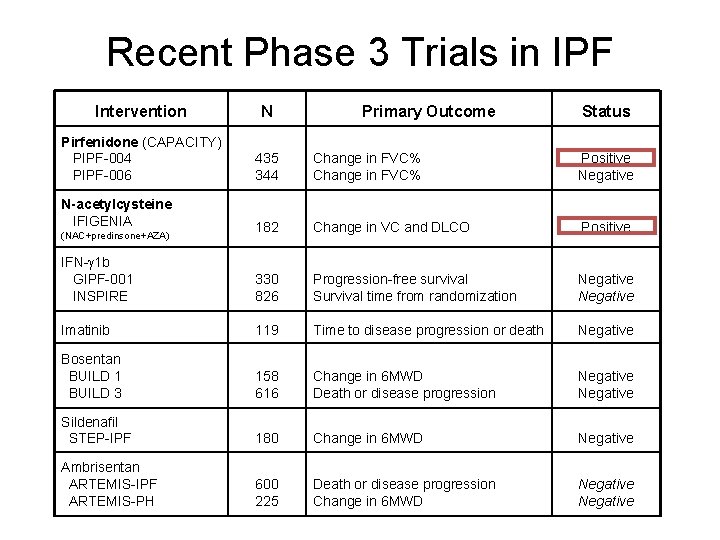
Recent Phase 3 Trials in IPF Intervention N Pirfenidone (CAPACITY) PIPF-004 PIPF-006 435 344 Change in FVC% Positive Negative N-acetylcysteine IFIGENIA 182 Change in VC and DLCO Positive IFN- 1 b GIPF-001 INSPIRE 330 826 Progression-free survival Survival time from randomization Negative Imatinib 119 Time to disease progression or death Negative Bosentan BUILD 1 BUILD 3 158 616 Change in 6 MWD Death or disease progression Negative Sildenafil STEP-IPF 180 Change in 6 MWD Negative Ambrisentan ARTEMIS-IPF ARTEMIS-PH 600 225 Death or disease progression Change in 6 MWD Negative (NAC+predinsone+AZA) Primary Outcome Status

Pirfenidone § Anti-fibrotic, anti-inflammatory and antioxidant properties § Approved for use in Japan, India and European Union in 2011 for treatment of mild-to-moderate IPF (FVC >50% predicted, DLCO > 35% predicted and 6 MWT distance > 150 m) § Tested in 4 multicenter trials (3 studies showed slowing of disease progression) Schaefer CJ, Eur Respir Rev, 2011 Noble PW et al, Lancet 2011

ASCEND Trial • Ongoing RDBPCT, phase 3 study to evaluate Pirfenidone in IPF • Enrolling patients in USA, Mexico, Australia, New Zealand South America • 500 patients, mid-2014 • Either Pirfenidone or placebo for one year (52 weeks)

Non Pharmacologic Therapies § Long-term oxygen therapy • One retrospective study, no survival benefit Douglas WW, AJRCCM 2000 • Indirect evidence from 2 large RCT’s in obstructive lung disease Ann Inter Med 1980, Lancet 1981 § Pulmonary rehabilitation • Two controlled trials showed an improvement in walk distance and symptom/quality of life Holland AE, Thorax 2008 Nishiyama et al, Respirology 2008

Lung Transplantation • Best therapeutic option for patients with severe IPF • Indications: ‒ Severe functional impairment- FVC < 60% predicted, DLCO < 40% predicted ‒ Nonresponsive to medical treatment with deterioration ‒ Hypoxemia • Logistics: ― Expertise ― waiting time

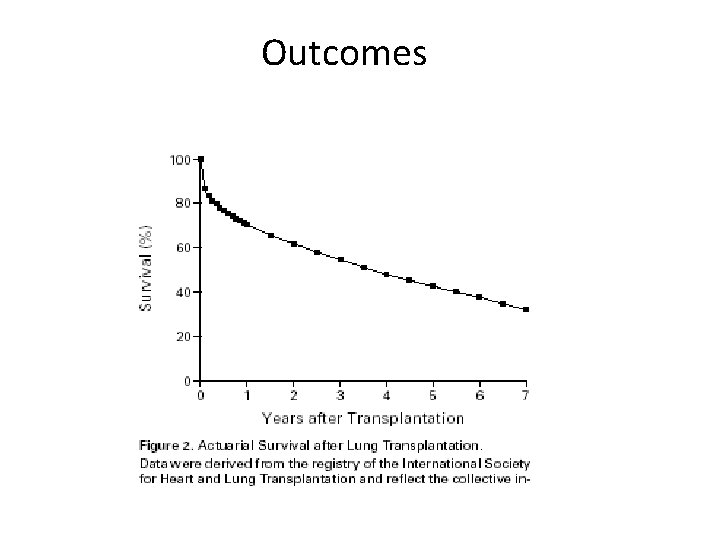
Outcomes

Cause of Death in IPF • 42 consecutive patients with IPF who had a post mortem at the Mayo Clinic (Rochester, MN, USA) over a 9 -yr period (1996 - 2004) • Respiratory failure (29%, major cause of death) • Other causes: PE, Lung cancer, Cardiac failure and CVA Daniels CE. Eur Resp J. 2008

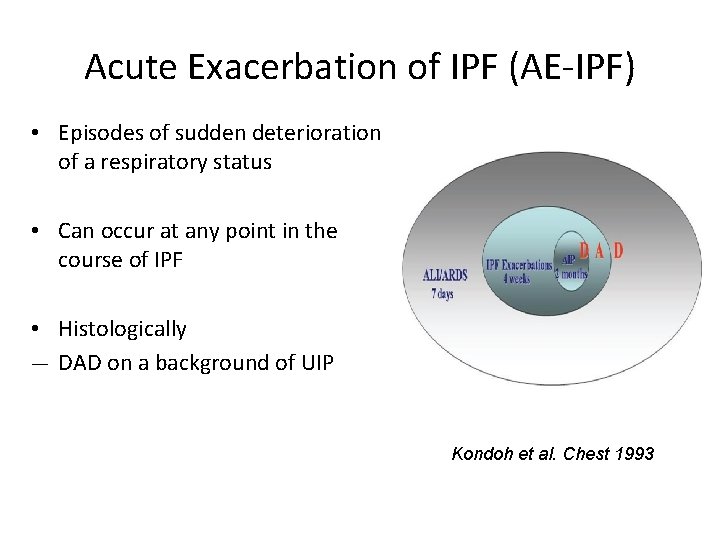
Acute Exacerbation of IPF (AE-IPF) • Episodes of sudden deterioration of a respiratory status • Can occur at any point in the course of IPF • Histologically ― DAD on a background of UIP Kondoh et al. Chest 1993

Acute Exacerbation of IPF (AE-IPF) ‘IPF Net Criteria’ • Previous or concurrent diagnosis of IPF • Worsening dyspnea of 30 days or less • New bilateral ground glass and/or consolidation (superimposed on IPF/UIP pattern on HRCT Chest) • No microbiological evidence of respiratory infection by endotracheal aspirate or BAL (if absent, can call ‘suspected acute exacerbation’) v Documentation of abnormal gas exchange has been removed Collard et al, AJRCCM 2007


Treatment of AE-IPF • Supportive care • Immunosuppressive therapy • High-dose corticosteroids • Commonly prescribed but no controlled trials • No recommendation about dose, route and duration of therapy • Tacrolimus • Cyclosporine Sakamota S et al, Intern Med 2010


AE-IPF • High mortality rate (90%) • Invasive mechanical ventilation is not recommended • Advance directives/end-of-life issues

Conclusion • IPF is a complex disease that progresses inexorably • Acute worsening is an important part of the natural history of disease • Diagnostic process is complex and requires multidisciplinary approach • IPF remains the most therapeutically challenging lung disease
