DIFFERENTIALS INTRODUCTION Given the appropriate specimens reagent and



























- Slides: 91

DIFFERENTIALS INTRODUCTION

Given the appropriate specimens, reagent, and equipment, perform four of five blood cell differentials and reticulocyte counts with no more than three instructor assists. (1) (2) (3) (4) (5) (6) (7) (8) Preparation of Peripheral Blood Smears Staining Techniques Rules for Cell Maturation WBC and Platelet Morphology RBC Morphology Reticulocytes Differential WBC Count Procedure

Preparation of Peripheral Blood Smear Used to perform differential white cell count Specimen Collection ◦ Venipuncture, fingerstick or heelstick ◦ EDTA whole blood less than four hours

Making Peripheral Blood Smears Place slide on gauze Use Diff-Safe dispenser or hematocrit tube Place blood 1/3 up from frosted end Use spreader slide

Making Peripheral Blood Smears h. Hold the spreader slide at a 30º angle h. Slide back toward the opposite end of the slide h. When contact is made, the specimen will spread along the spreader slide

Push the spreader slide toward the opposite end of the slide

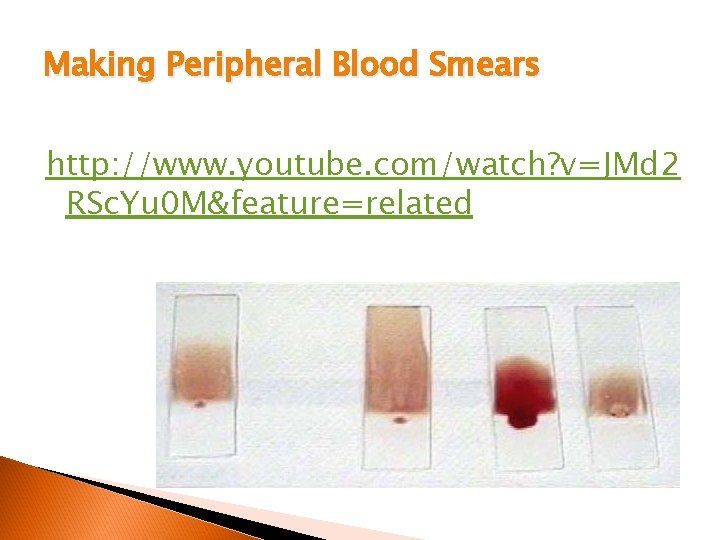
Making Peripheral Blood Smears http: //www. youtube. com/watch? v=JMd 2 RSc. Yu 0 M&feature=related

Criteria Feathered edge l NO lines, ridges, or holes l Length of smear - no more then 2/3 the length of the slide l Length too long - decrease specimen, increase angle l Length too short - increase specimen, decrease angle l Speed l

Staining Techniques Wright’s Stain ◦ Methyl alcohol - Fixative ◦ Methylene blue – Nucleus ◦ Eosin - Cytoplasm

Staining Techniques Modified Wright’s Stain ◦ Glycerin – Freezing RBCs ◦ Giemsa - Parasites

Stain Buffers Stains must have a p. H of 6. 4 ◦ Too acidic: RBCs appear bright red WBC nucleus appears pale blue or colorless

Stain Buffers ◦ Too alkaline: RBCs appear grey or deep blue WBCs appear very dark blueblack

Staining Methods Stain Jar – excellent for STAT specimens Automatic – excellent for large volumes

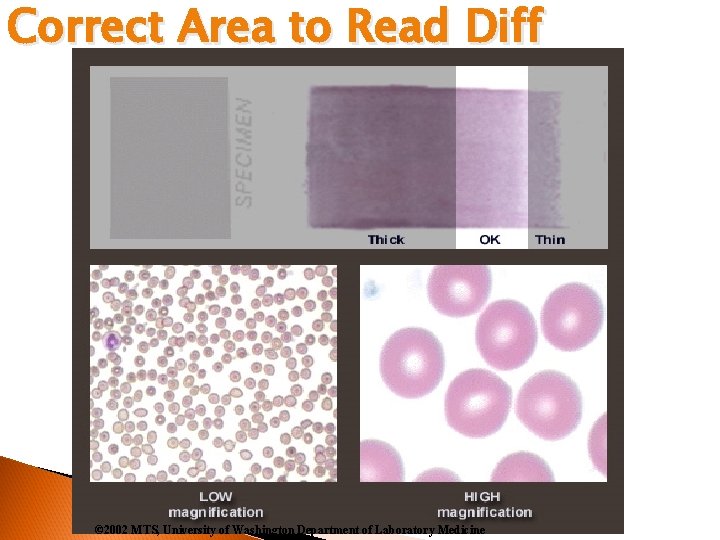
Correct Area to Read Diff © 2002 MTS, University of Washington Department of Laboratory Medicine

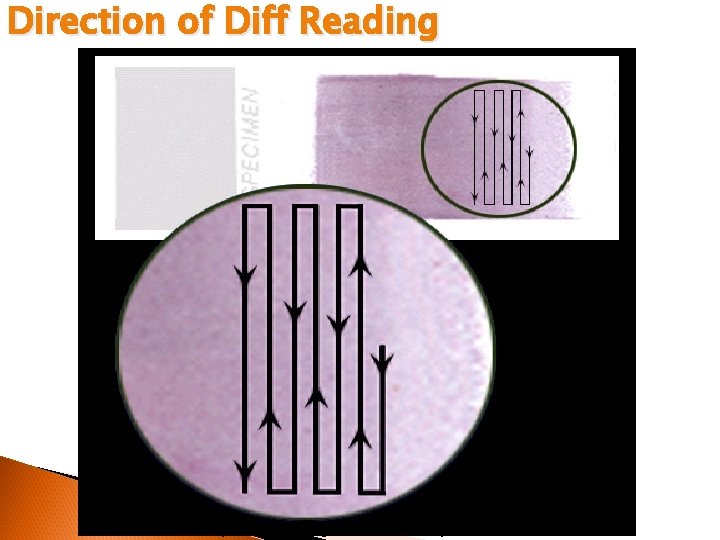
Direction of Diff Reading © 2002 MTS, University of Washington Department of Laboratory Medicine

Rules of Maturation Size l Cytoplasm O Percentage O Color O Granules l Nucleus - Shape l

WBC and Platelet Morphology LEUKOPOIESIS: The production of WBCs Develop Two in Bone Marrow groups of WBCs ◦ Granulocytes ◦ Agranulocytes

Maturation sequence of granulocytes Myeloblast l Promyelocyte l Metamyelocyte (Neutrophilic) l Band Neutrophil (Stab) l Segmented Neutrophil l

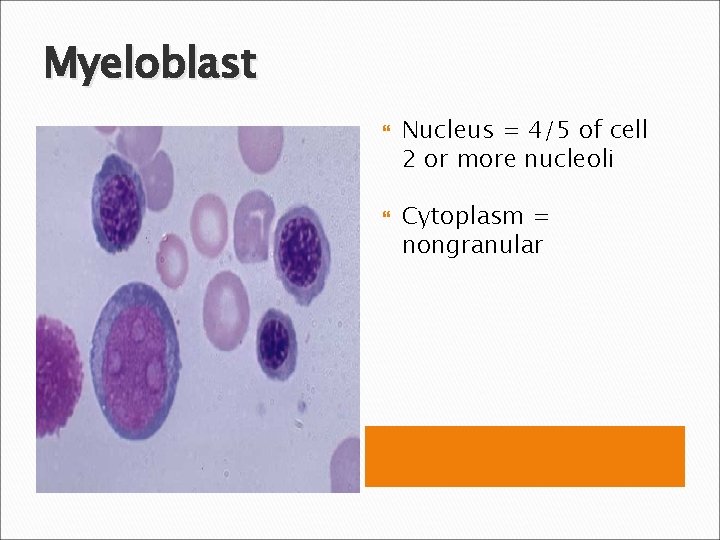
Myeloblast Nucleus = 4/5 of cell 2 or more nucleoli Cytoplasm = nongranular

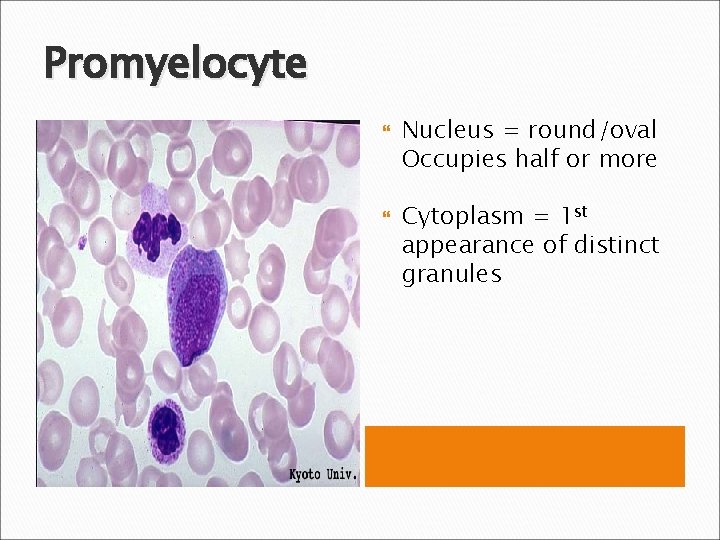
Promyelocyte Nucleus = round/oval Occupies half or more Cytoplasm = 1 st appearance of distinct granules

Myelocyte Last stage capable of cell division Nucleus = slightly flattened on one side no nucleoli Cytoplasm = “Dawn of Neutrophilia”

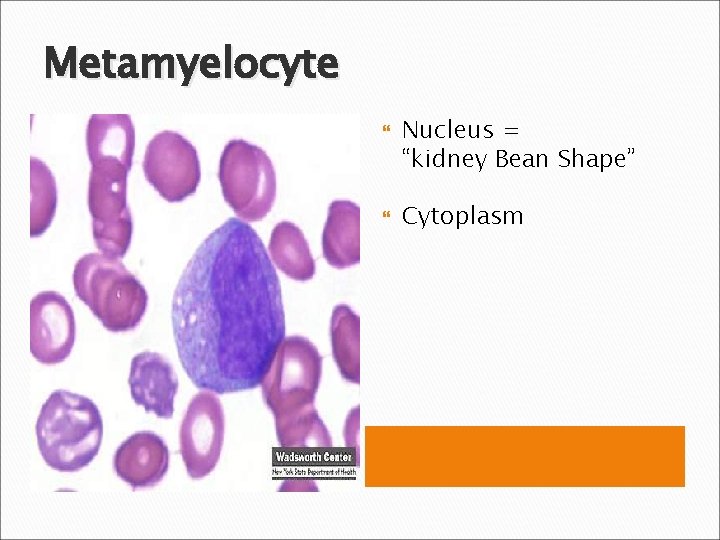
Metamyelocyte Nucleus = “kidney Bean Shape” Cytoplasm

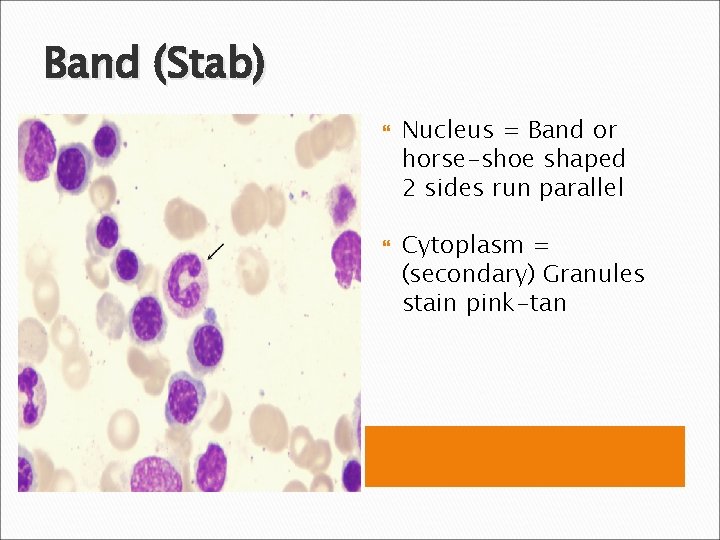
Band (Stab) Nucleus = Band or horse-shoe shaped 2 sides run parallel Cytoplasm = (secondary) Granules stain pink-tan

Segmented Neutrophil Nucleus = 2 -5 segments (lobes) connected by threadlike filaments Cytoplasm = Stains pink-tan (secondary) granules Highest % of WBC seen Bacterial Infections

Eosinophil Nucleus = Usually 2 lobes Cytoplasm = Large Orange to bright red. “berry shaped granules Allergies & Parasitic Infections

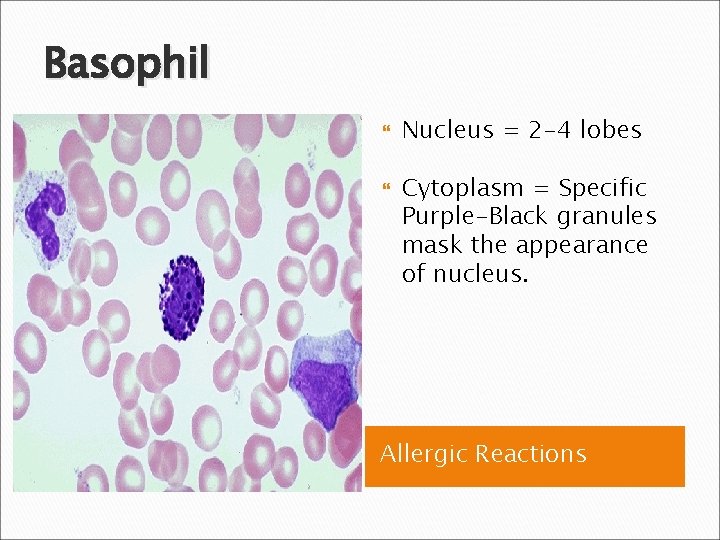
Basophil Nucleus = 2 -4 lobes Cytoplasm = Specific Purple-Black granules mask the appearance of nucleus. Allergic Reactions

Agranulocytic Series Agranulocytes are placed into three groups: ◦ Lymphocytic series Lymphocytes ◦ Monocytic series Monocyte ◦ Plasmocytic series Plamocyte

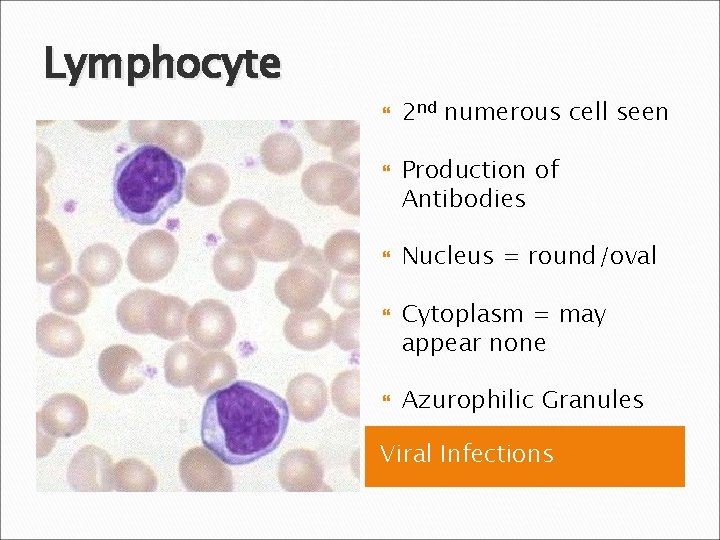
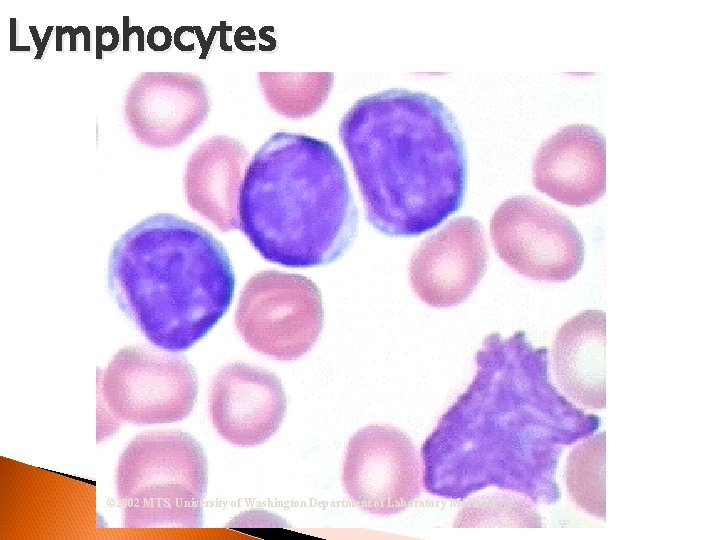
Lymphocyte 2 nd numerous cell seen Production of Antibodies Nucleus = round/oval Cytoplasm = may appear none Azurophilic Granules Viral Infections

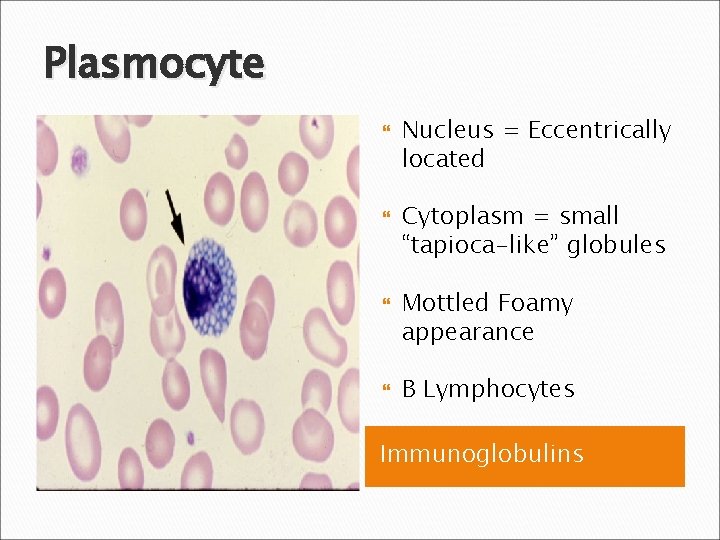
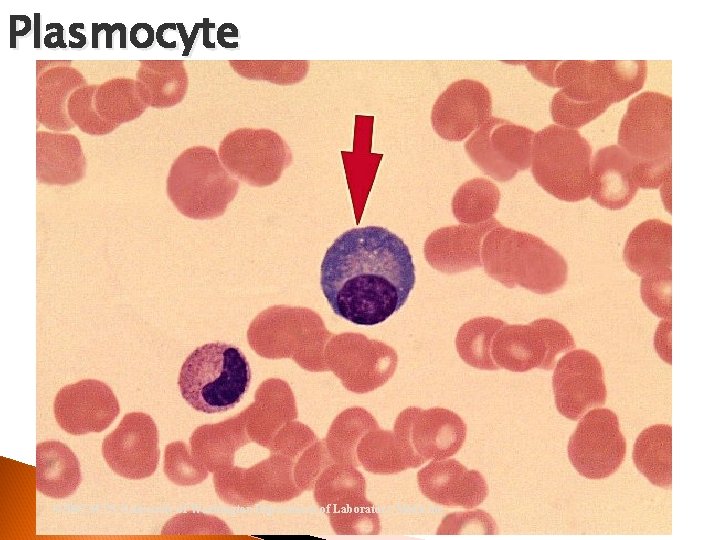
Plasmocyte Nucleus = Eccentrically located Cytoplasm = small “tapioca-like” globules Mottled Foamy appearance B Lymphocytes Immunoglobulins

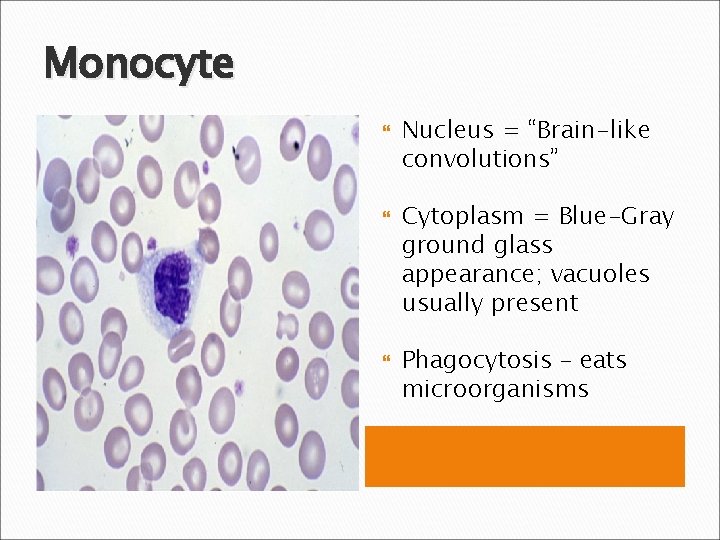
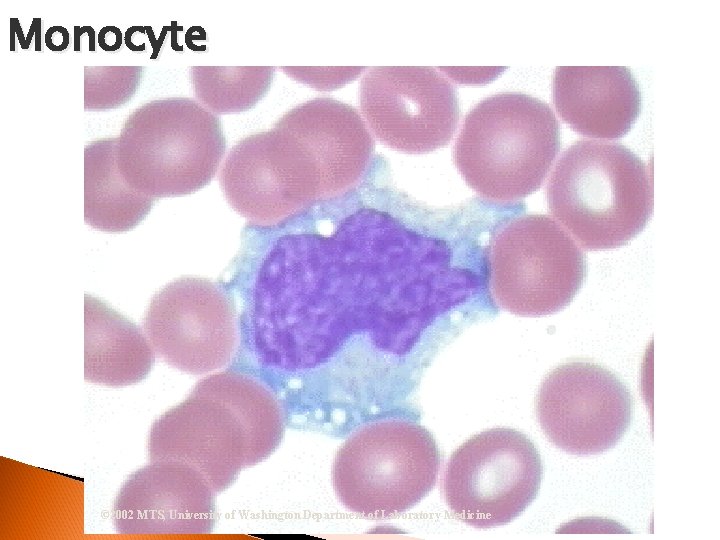
Monocyte Nucleus = “Brain-like convolutions” Cytoplasm = Blue-Gray ground glass appearance; vacuoles usually present Phagocytosis – eats microorganisms

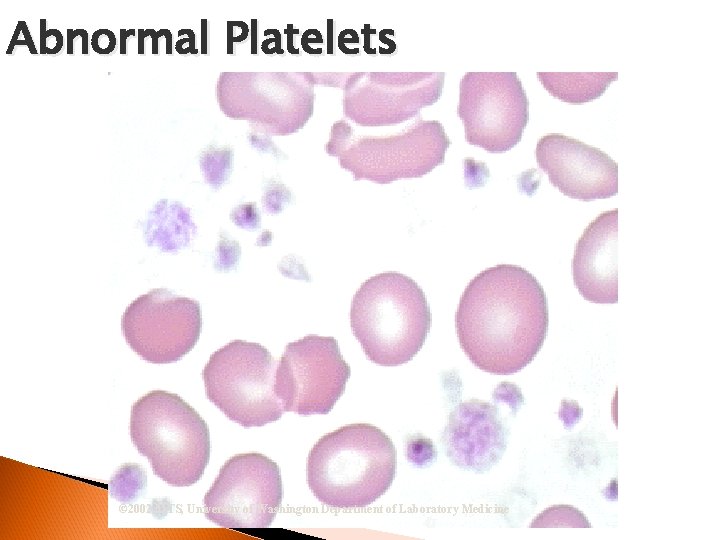
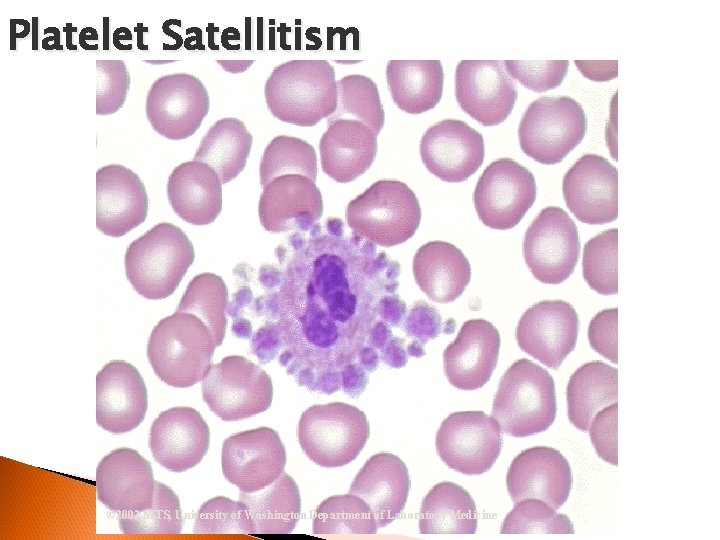
Thrombocytes (Platelet) Important in the coagulation process Less mature stages rarely seen Maturation sequence: Abnormal ◦ Megakaryoblast Platelets ◦ Promegakaryocyte ◦ Megakaryocyte ◦ Thrombocyte Platelet Satellitism

Thrombocyte (platelet) Coagulation Process Produced directly from the cytoplasm of Megakaryocytes Round, oval, or spindle shaped Platelets

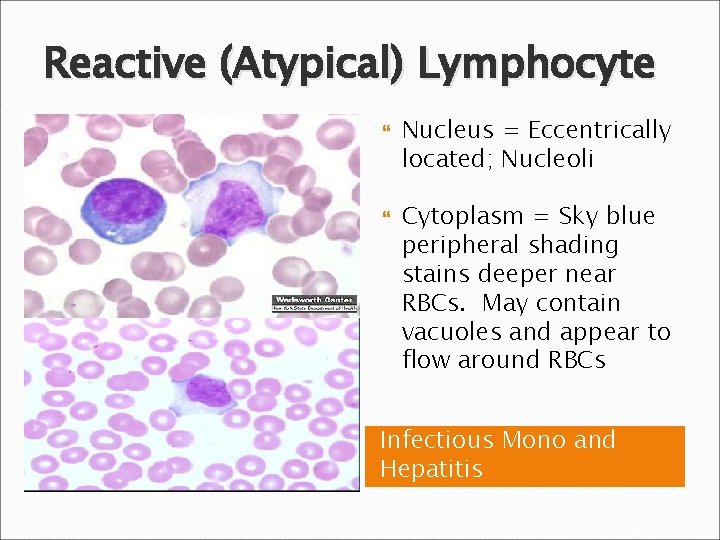
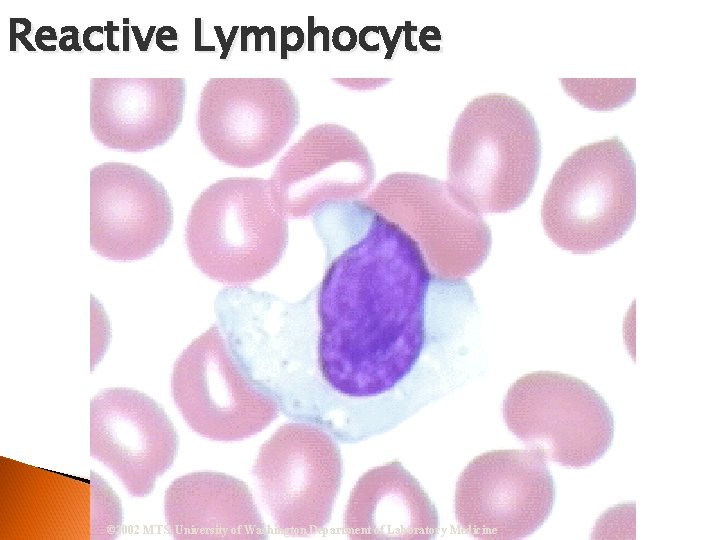
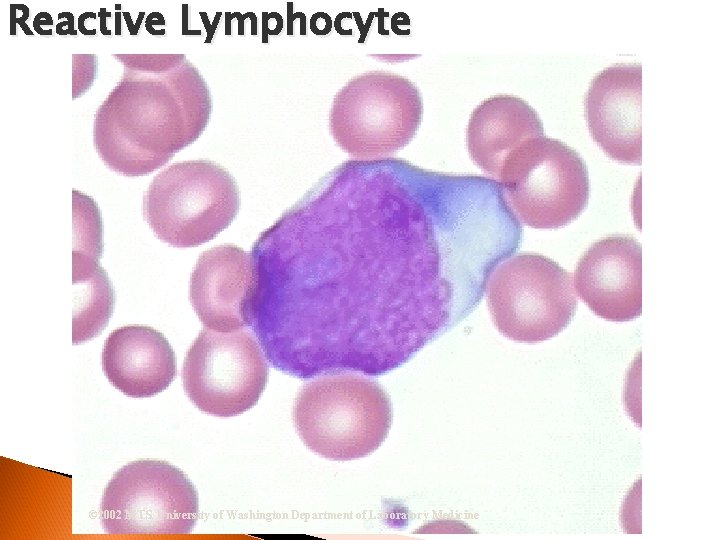
Reactive (Atypical) Lymphocyte Nucleus = Eccentrically located; Nucleoli Cytoplasm = Sky blue peripheral shading stains deeper near RBCs. May contain vacuoles and appear to flow around RBCs Infectious Mono and Hepatitis

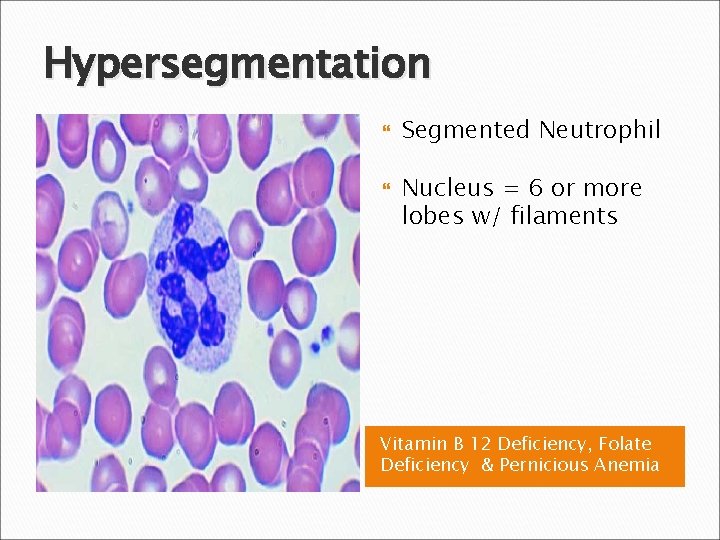
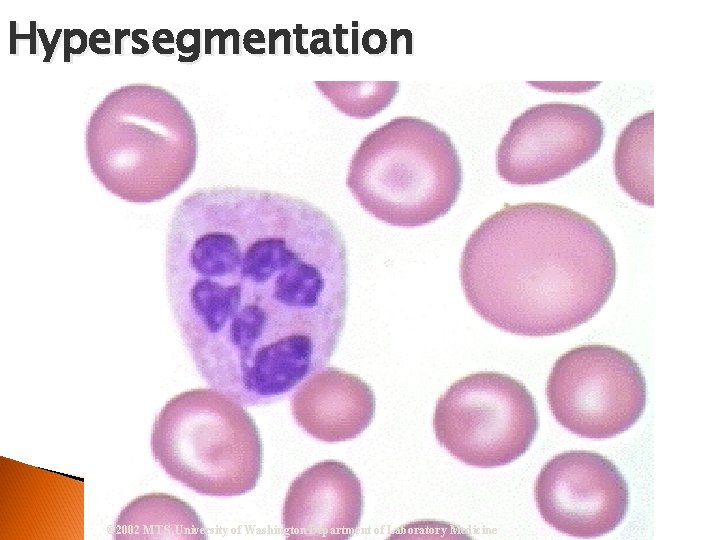
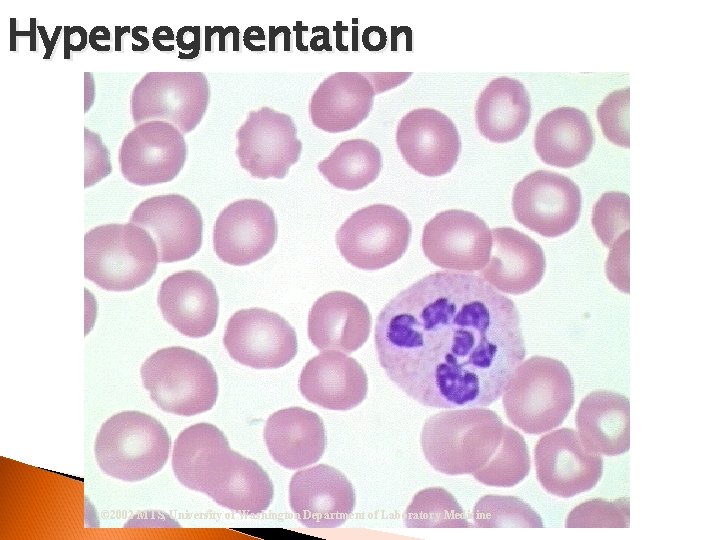
Hypersegmentation Segmented Neutrophil Nucleus = 6 or more lobes w/ filaments Vitamin B 12 Deficiency, Folate Deficiency & Pernicious Anemia

Pelger-Huet Anomaly Two-lobe connected by a filament (Pince-Nez) Or One-Lobed (peanutshaped)

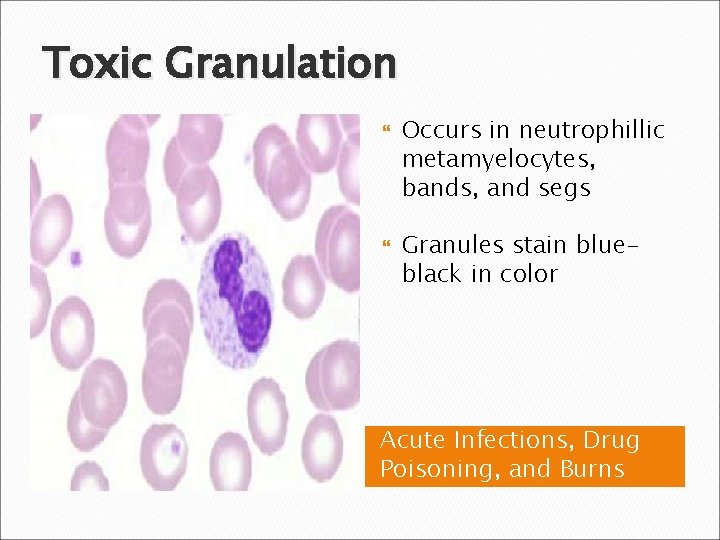
Toxic Granulation Occurs in neutrophillic metamyelocytes, bands, and segs Granules stain blueblack in color Acute Infections, Drug Poisoning, and Burns

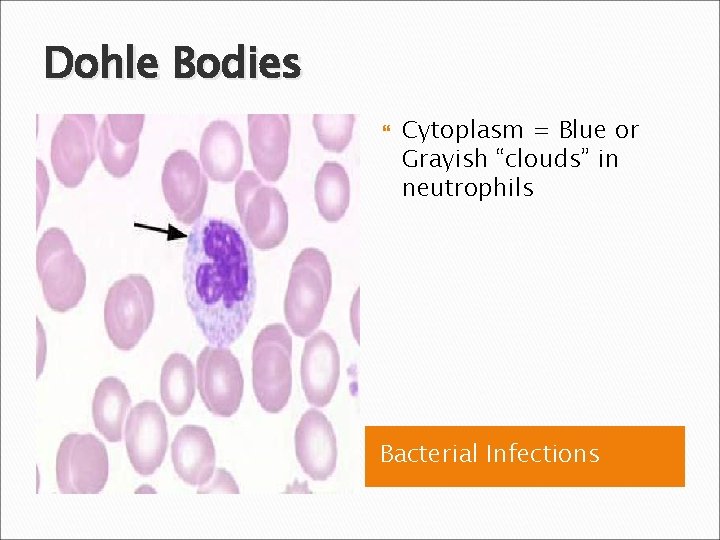
Dohle Bodies Cytoplasm = Blue or Grayish “clouds” in neutrophils Bacterial Infections

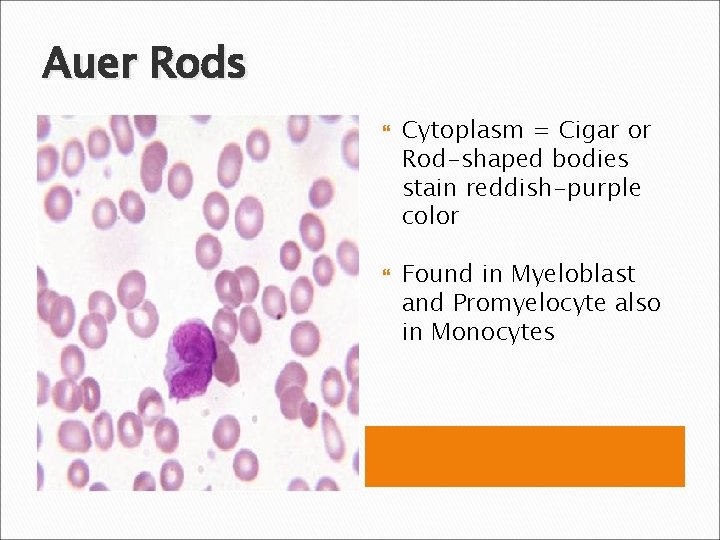
Auer Rods Cytoplasm = Cigar or Rod-shaped bodies stain reddish-purple color Found in Myeloblast and Promyelocyte also in Monocytes

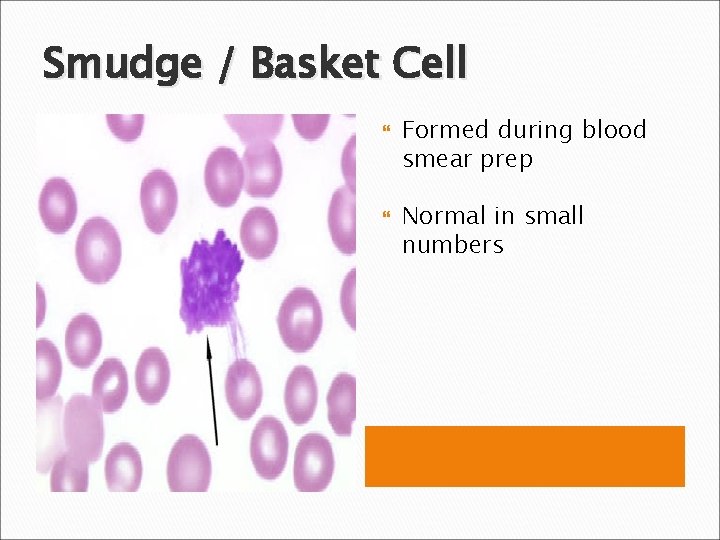
Smudge / Basket Cell Formed during blood smear prep Normal in small numbers

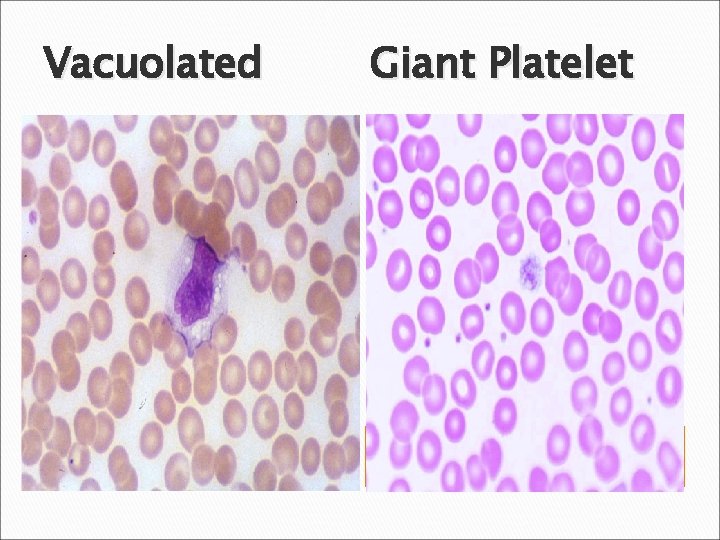
Vacuolated Giant Platelet

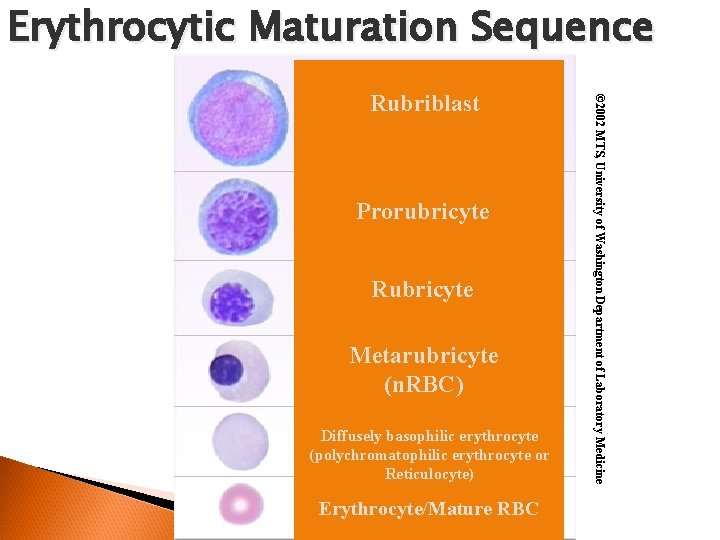
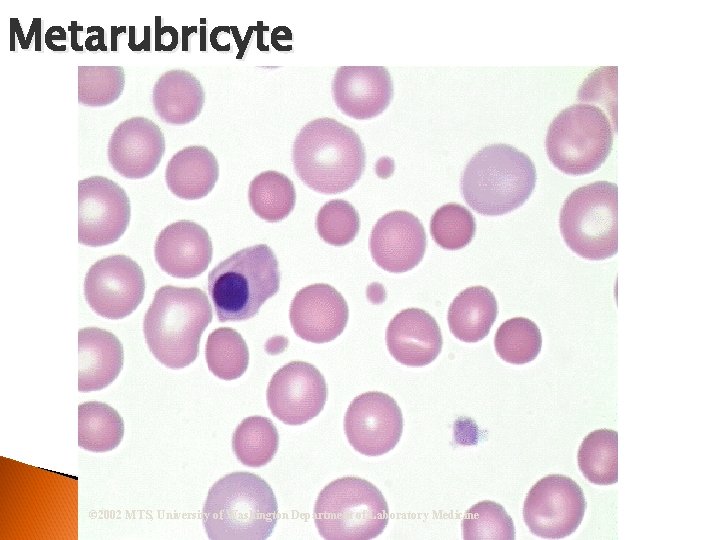
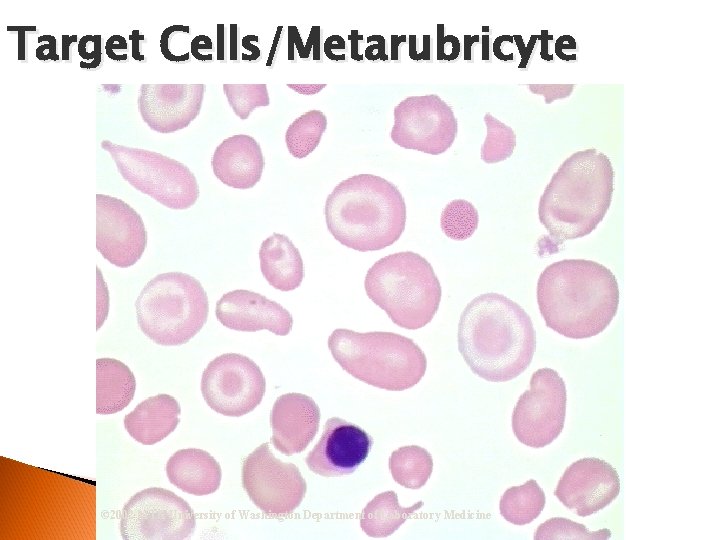
Erythrocytic Maturation Sequence Prorubricyte Rubricyte Metarubricyte (n. RBC) Diffusely basophilic erythrocyte (polychromatophilic erythrocyte or Reticulocyte) Erythrocyte/Mature RBC © 2002 MTS, University of Washington Department of Laboratory Medicine Rubriblast

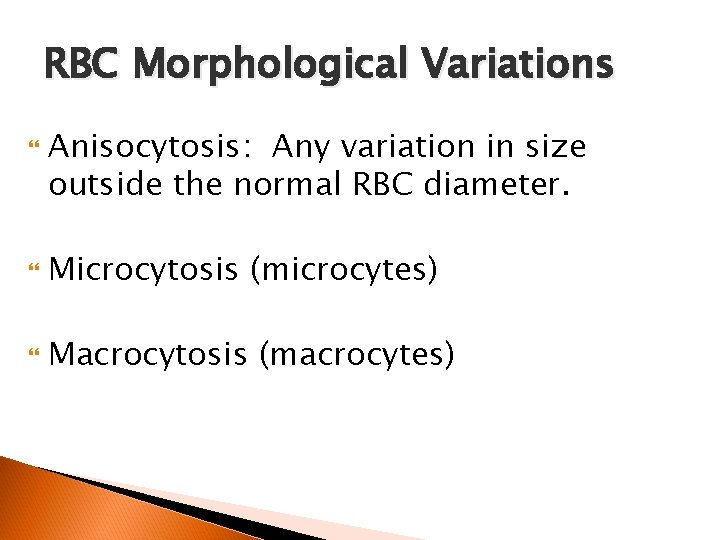
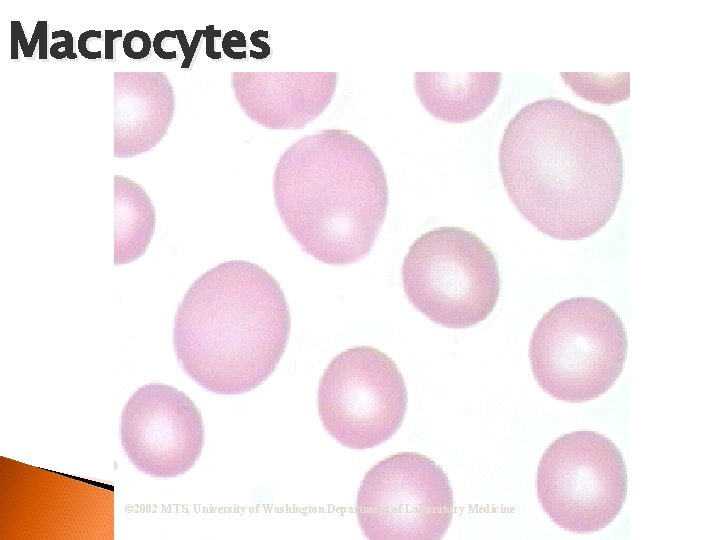
RBC Morphological Variations Anisocytosis: Any variation in size outside the normal RBC diameter. Microcytosis (microcytes) Macrocytosis (macrocytes)

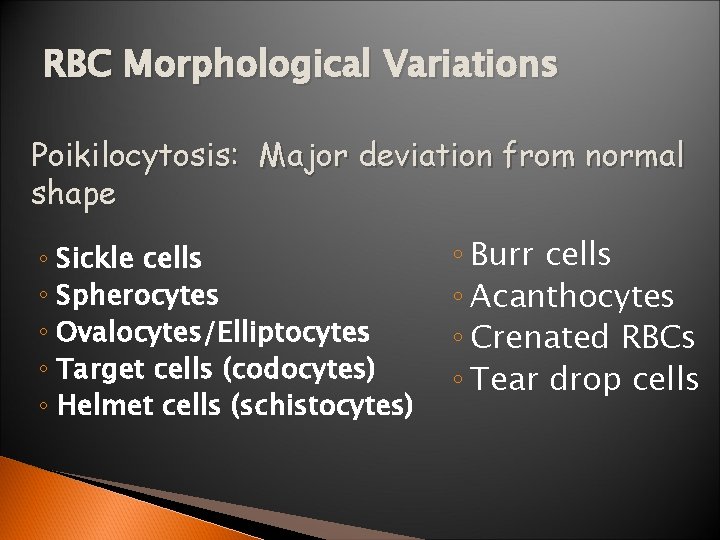
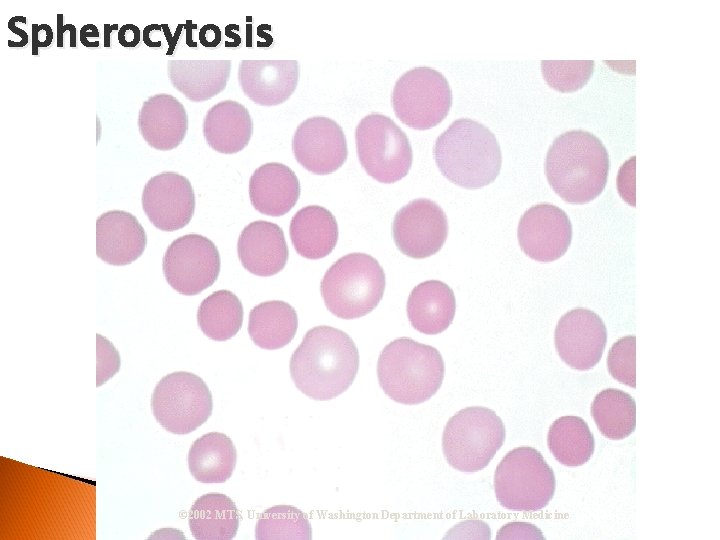
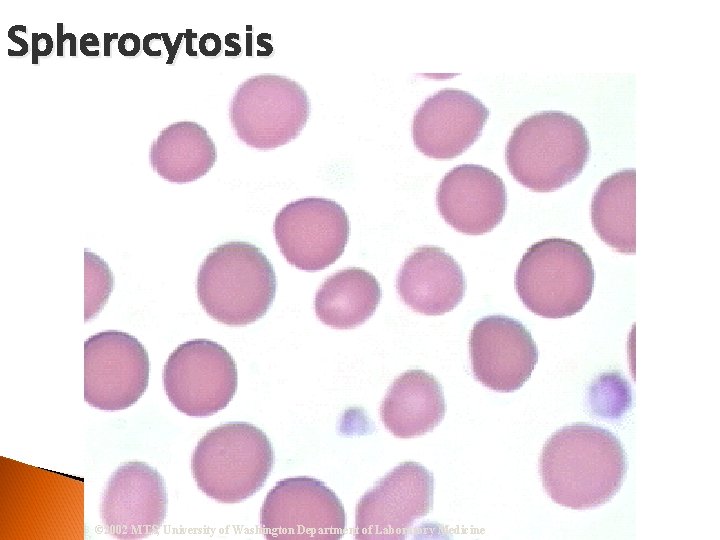
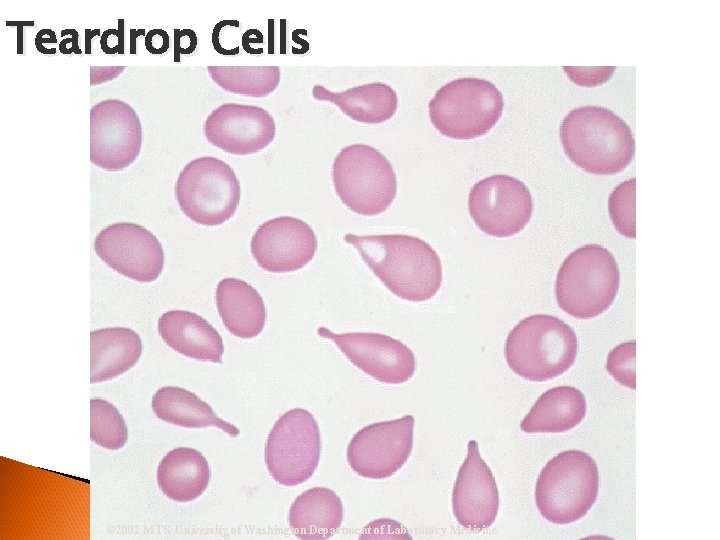
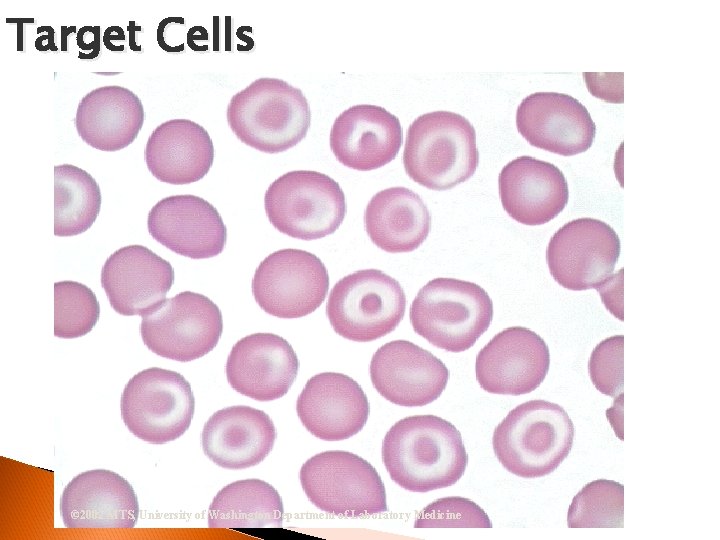
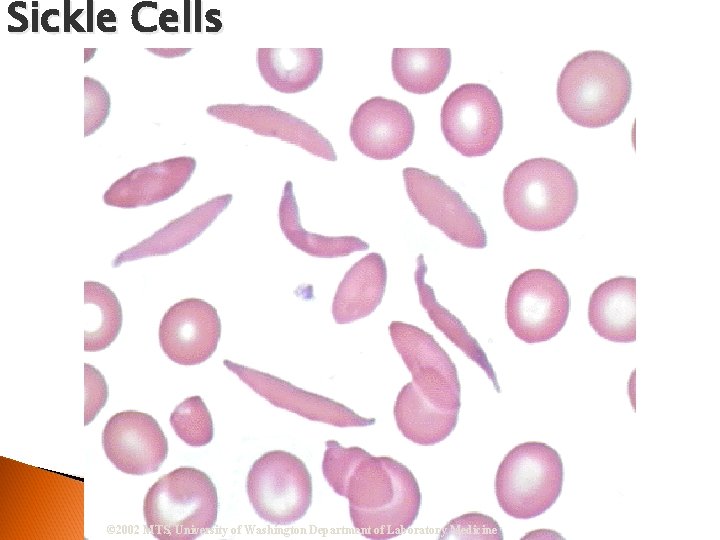
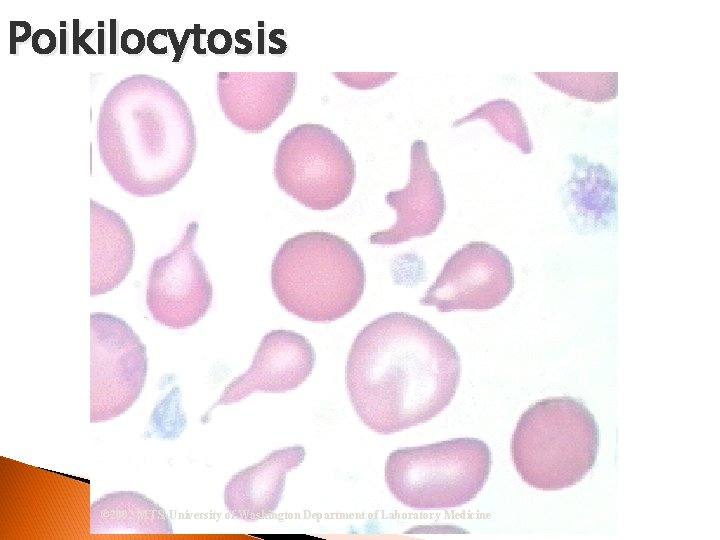
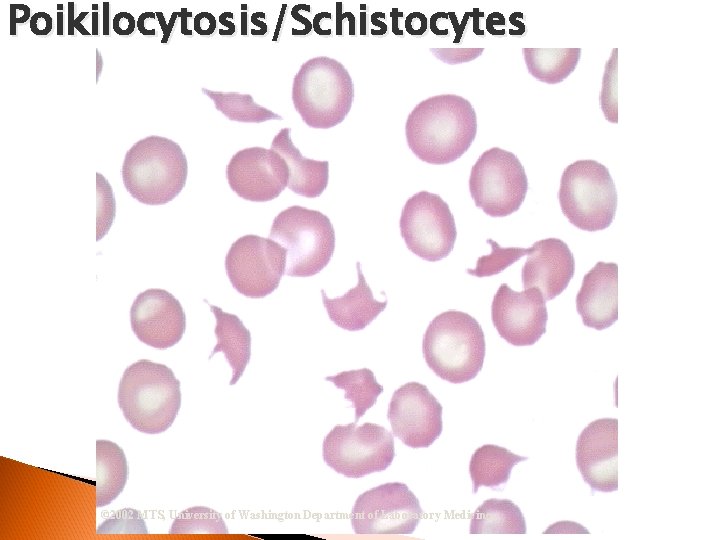
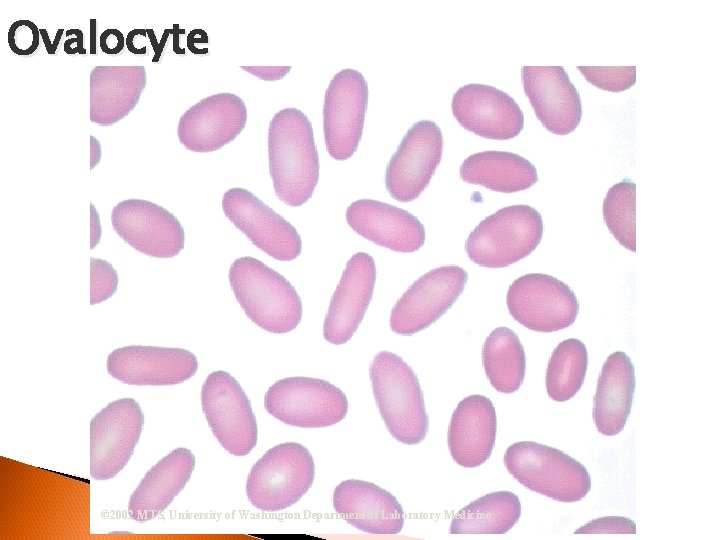
RBC Morphological Variations Poikilocytosis: Major deviation from normal shape ◦ Sickle cells ◦ Spherocytes ◦ Ovalocytes/Elliptocytes ◦ Target cells (codocytes) ◦ Helmet cells (schistocytes) ◦ Burr cells ◦ Acanthocytes ◦ Crenated RBCs ◦ Tear drop cells

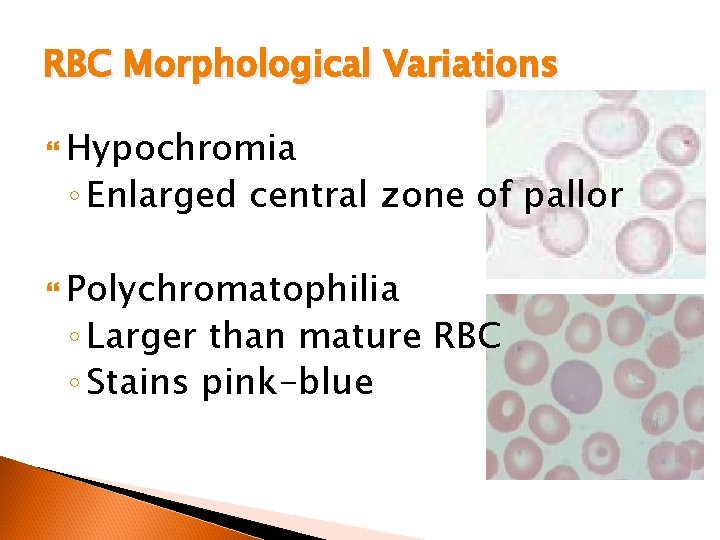
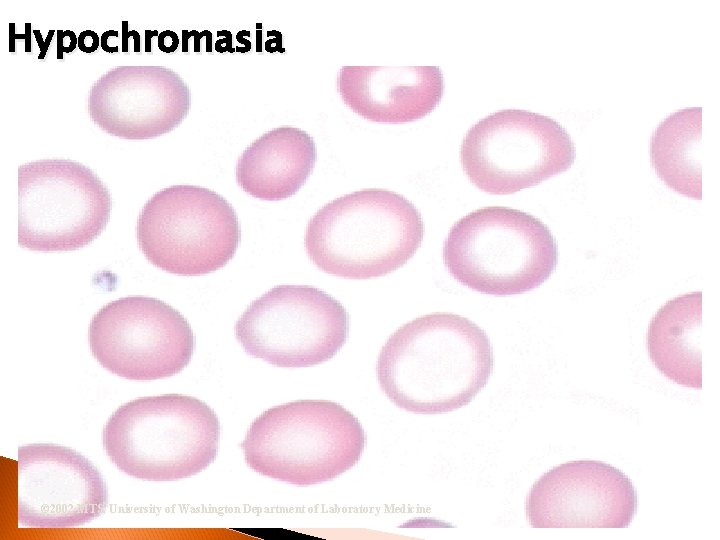
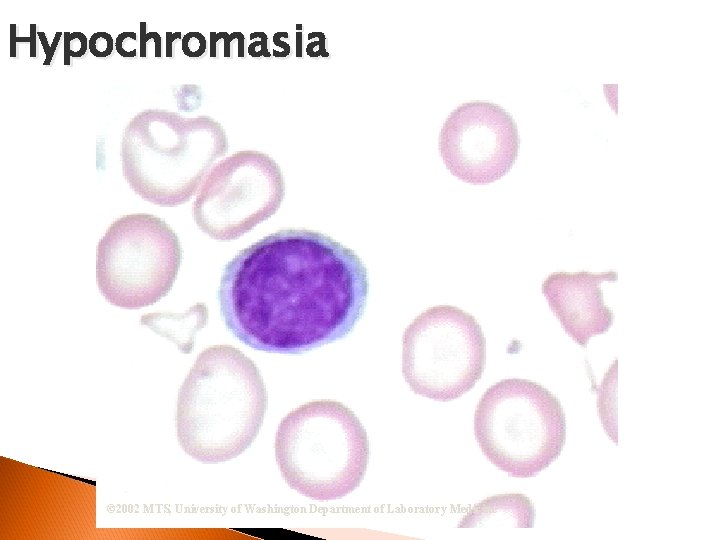
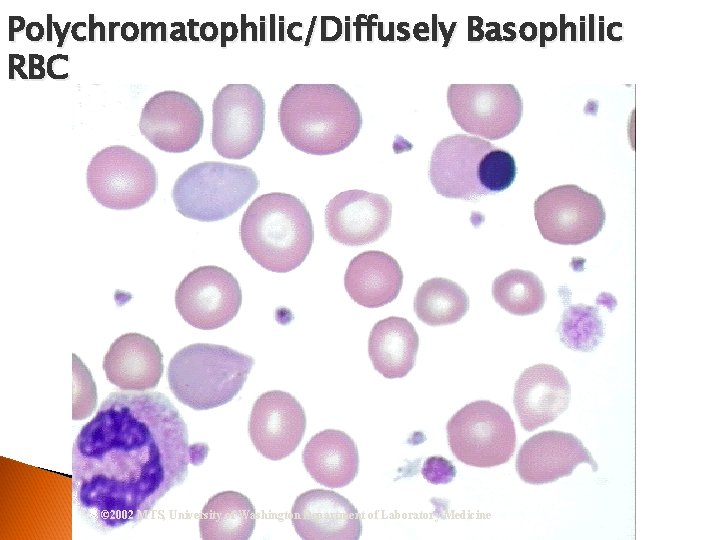
RBC Morphological Variations Hypochromia ◦ Enlarged central zone of pallor Polychromatophilia ◦ Larger than mature RBC ◦ Stains pink-blue

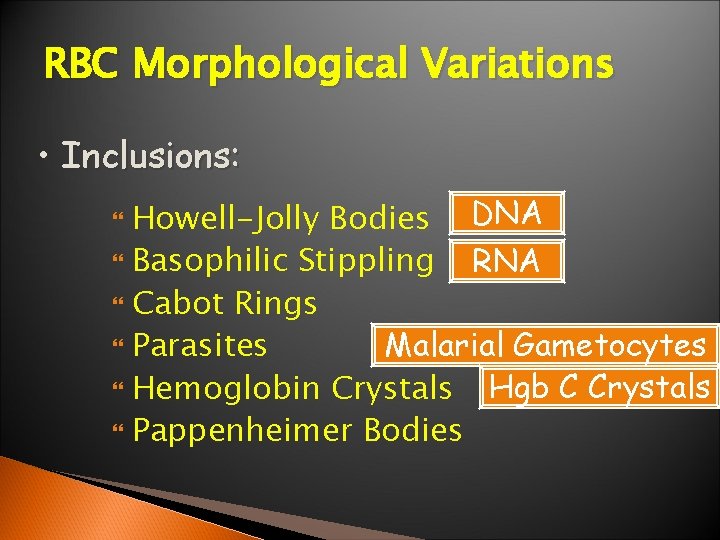
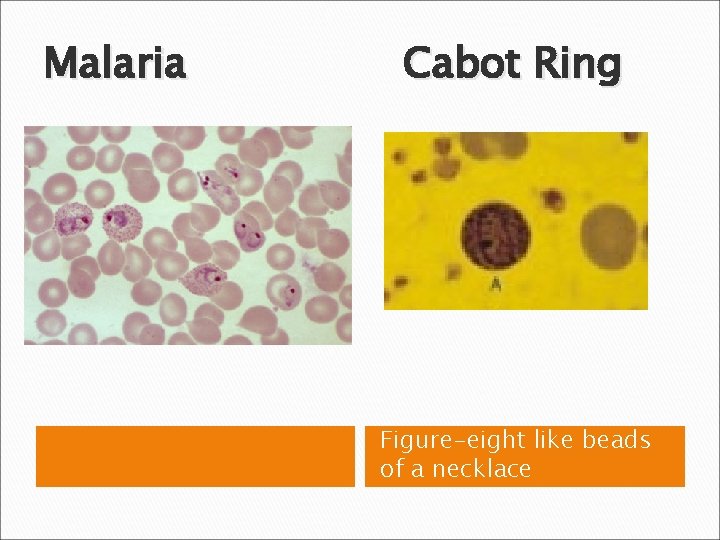
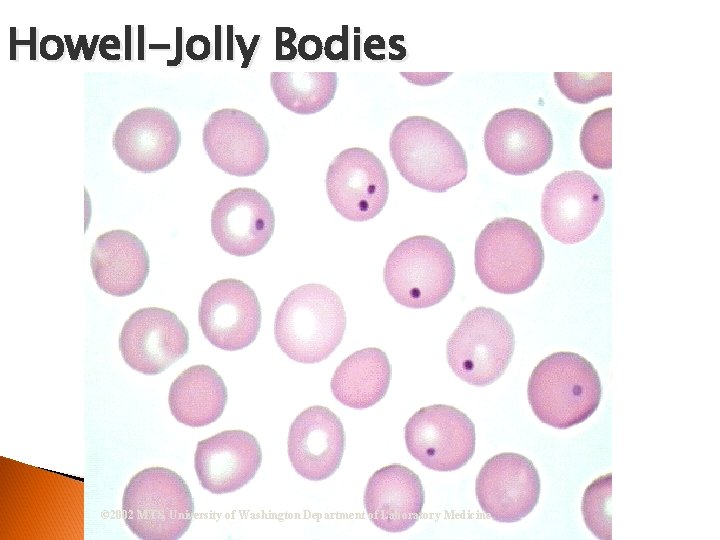
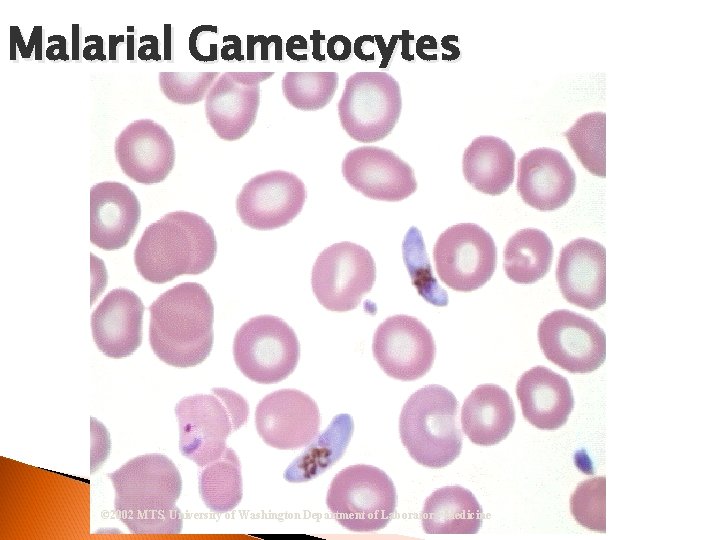
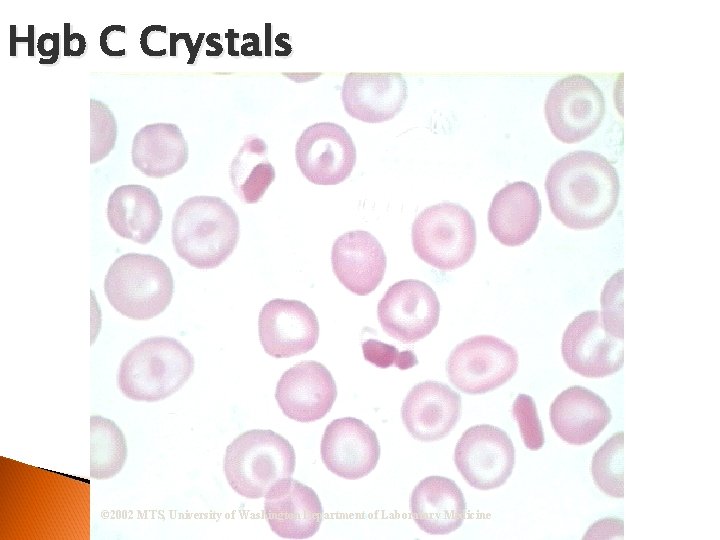
RBC Morphological Variations • Inclusions: Howell-Jolly Bodies DNA Basophilic Stippling RNA Cabot Rings Malarial Gametocytes Parasites Hgb C Crystals Hemoglobin Crystals Pappenheimer Bodies

Malaria Cabot Ring Figure-eight like beads of a necklace

3 c. Using the procedure, specimens, reagents, and equipment, perform three blood cell differentials with no more than four instructor assists.

Examination of blood smear Quantitative and qualitative analysis of WBCs Semi-quantitative and qualitative analysis of platelets Evaluation of the morphological characteristics of RBCs

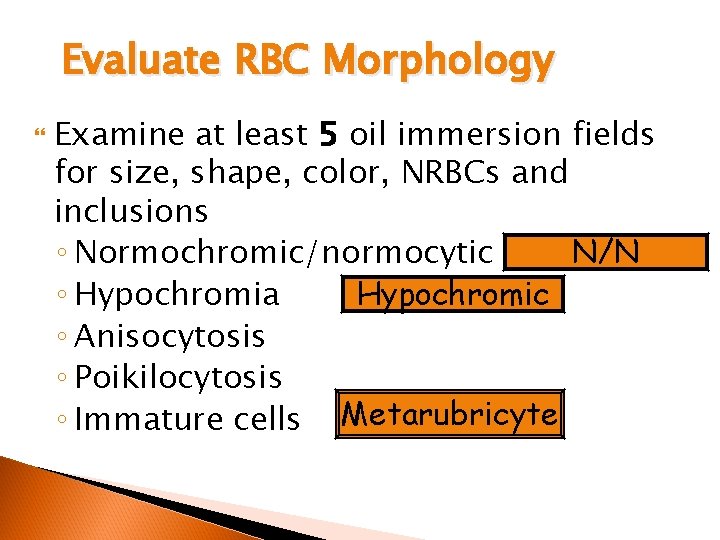
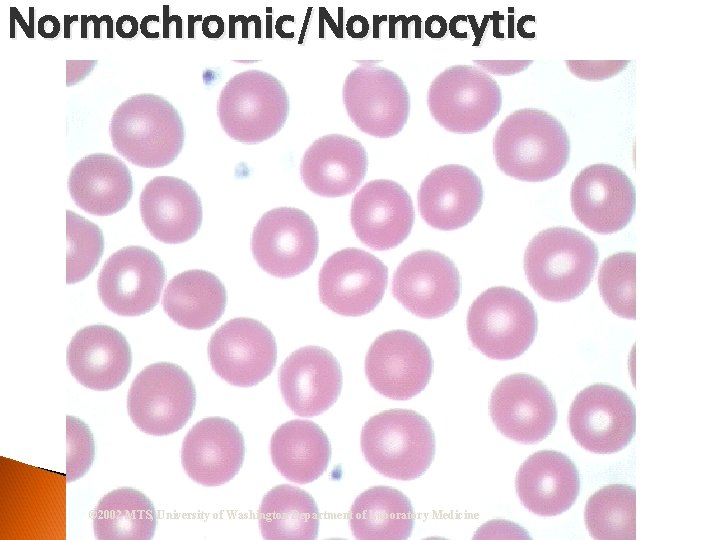
Evaluate RBC Morphology Examine at least 5 oil immersion fields for size, shape, color, NRBCs and inclusions N/N ◦ Normochromic/normocytic ◦ Hypochromia Hypochromic ◦ Anisocytosis ◦ Poikilocytosis ◦ Immature cells Metarubricyte

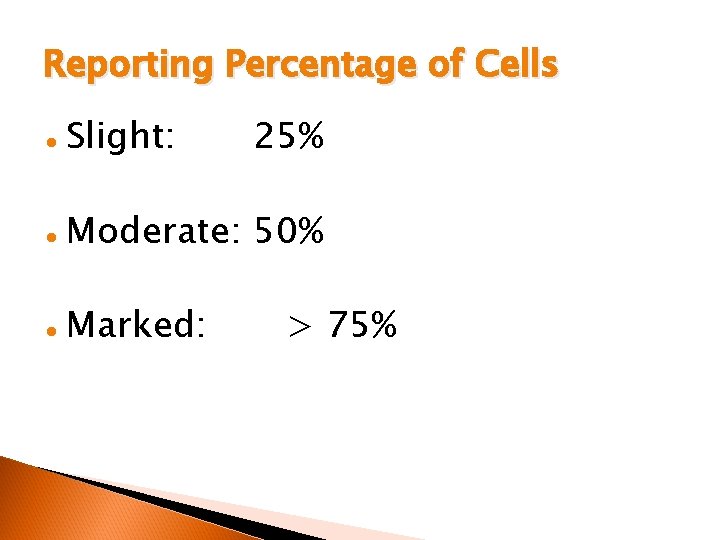
Reporting Percentage of Cells l Slight: 25% l Moderate: 50% l Marked: > 75%

Platelet Estimation 0 -6 platelets/oil immersion field ◦ Report as decreased 7 -20 platelets/oil immersion field ◦ Report as adequate >20 platelets/oil immersion field ◦ Report as increased

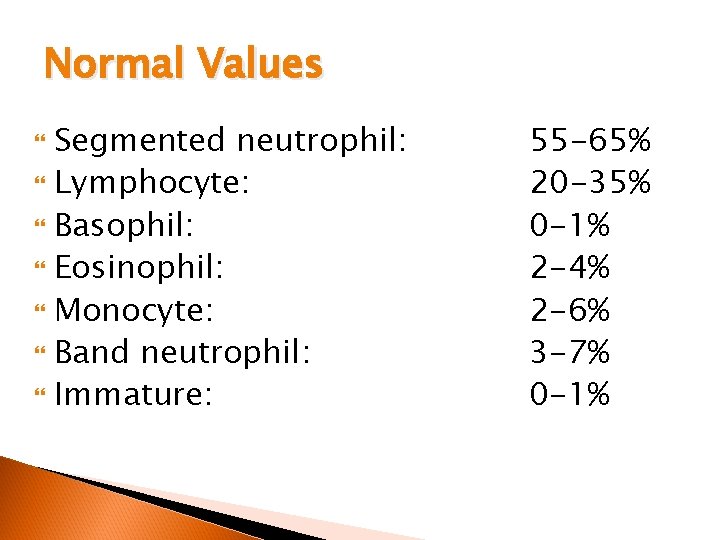
Normal Values Segmented neutrophil: Lymphocyte: Basophil: Eosinophil: Monocyte: Band neutrophil: Immature: 55 -65% 20 -35% 0 -1% 2 -4% 2 -6% 3 -7% 0 -1%

3 d. Using the procedure, specimens, reagents, and equipment, perform reticulocyte counts with no more than four instructor assists.

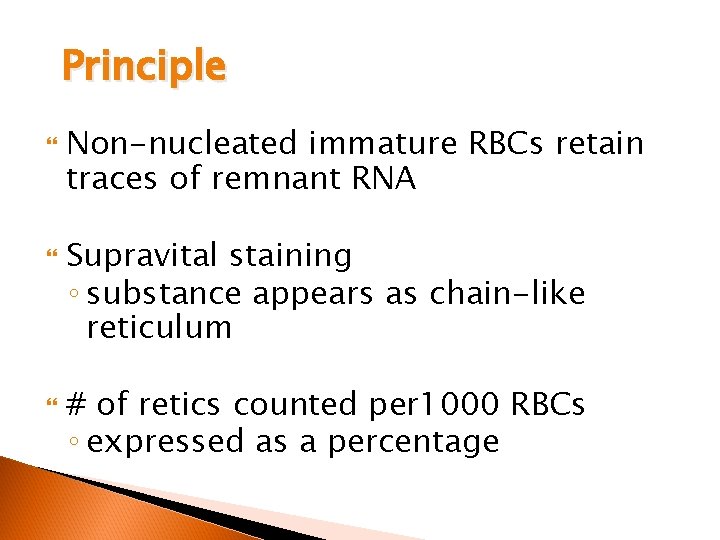
Principle Non-nucleated immature RBCs retain traces of remnant RNA Supravital staining ◦ substance appears as chain-like reticulum # of retics counted per 1000 RBCs ◦ expressed as a percentage

Calculations % Reticulocytes = Total # retics per 1000 RBCs 10

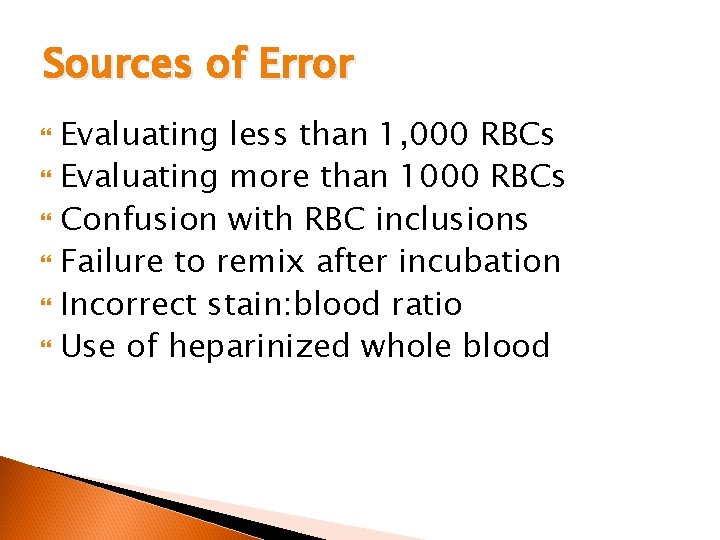
Sources of Error Evaluating less than 1, 000 RBCs Evaluating more than 1000 RBCs Confusion with RBC inclusions Failure to remix after incubation Incorrect stain: blood ratio Use of heparinized whole blood

3 a. Using the procedure, specimens, and equipment, prepare two peripheral blood smears with a feathered edge covering one-half to two-thirds the length of the microscope slide. (1) Preparation of Peripheral Blood Smears (2) Staining Techniques

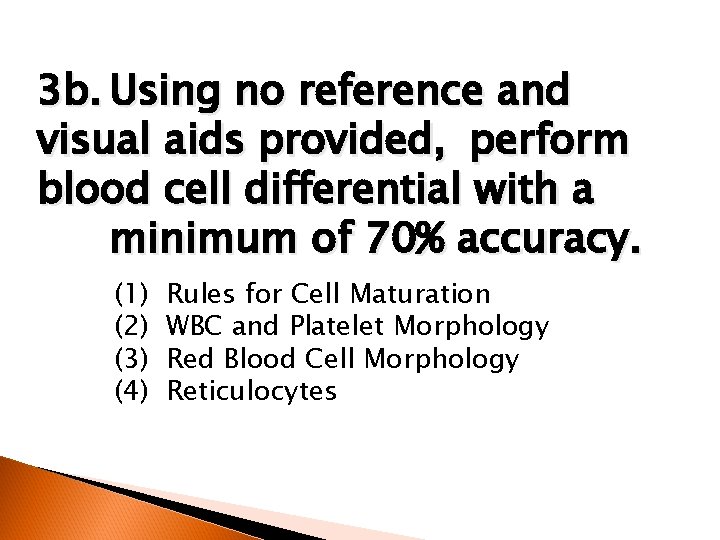
3 b. Using no reference and visual aids provided, perform blood cell differential with a minimum of 70% accuracy. (1) (2) (3) (4) Rules for Cell Maturation WBC and Platelet Morphology Red Blood Cell Morphology Reticulocytes

3 c. Using the procedure, specimens, reagents, and equipment, perform blood cell differentials with no more than four instructor assists. (1) Differential White Blood Cell Count (2) Normal Values

3 d. Using the procedure, specimens, reagents, and equipment, perform reticulocyte counts with no more than four instructor assists. (1) (2) (3) (4) (5) (6) (7) (8) Clinical Significance Principle Equipment, Reagents, and Supplies Procedure Calculations Normal Values Sources of Error ORM Group Discussion

Hypochromasia © 2002 MTS, University of Washington Department of Laboratory Medicine

Spherocytosis © 2002 MTS, University of Washington Department of Laboratory Medicine

Spherocytosis © 2002 MTS, University of Washington Department of Laboratory Medicine

Teardrop Cells © 2002 MTS, University of Washington Department of Laboratory Medicine

Target Cells © 2002 MTS, University of Washington Department of Laboratory Medicine

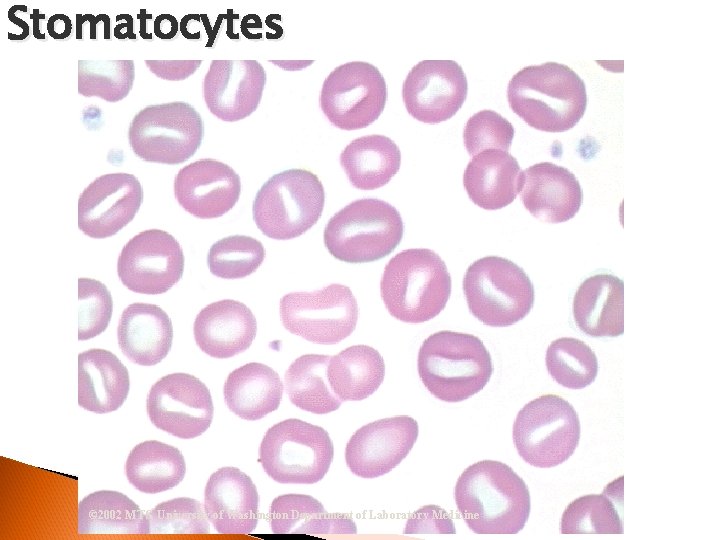
Stomatocytes © 2002 MTS, University of Washington Department of Laboratory Medicine

Sickle Cells © 2002 MTS, University of Washington Department of Laboratory Medicine

Basophilic Stippling © 2002 MTS, University of Washington Department of Laboratory Medicine

Reactive Lymphocyte © 2002 MTS, University of Washington Department of Laboratory Medicine

Reactive Lymphocyte © 2002 MTS, University of Washington Department of Laboratory Medicine

Poikilocytosis © 2002 MTS, University of Washington Department of Laboratory Medicine

Poikilocytosis/Schistocytes © 2002 MTS, University of Washington Department of Laboratory Medicine

Abnormal Platelets © 2002 MTS, University of Washington Department of Laboratory Medicine

Platelet Satellitism © 2002 MTS, University of Washington Department of Laboratory Medicine

Plasmocyte © 2002 MTS, University of Washington Department of Laboratory Medicine

Metarubricyte © 2002 MTS, University of Washington Department of Laboratory Medicine

Normochromic/Normocytic © 2002 MTS, University of Washington Department of Laboratory Medicine

Monocyte © 2002 MTS, University of Washington Department of Laboratory Medicine

Lymphocytes © 2002 MTS, University of Washington Department of Laboratory Medicine

Hypochromasia © 2002 MTS, University of Washington Department of Laboratory Medicine

Hypersegmentation © 2002 MTS, University of Washington Department of Laboratory Medicine

Hypersegmentation © 2002 MTS, University of Washington Department of Laboratory Medicine

Ovalocyte © 2002 MTS, University of Washington Department of Laboratory Medicine

Macrocytes © 2002 MTS, University of Washington Department of Laboratory Medicine

Burr Cells © 2002 MTS, University of Washington Department of Laboratory Medicine

Howell-Jolly Bodies © 2002 MTS, University of Washington Department of Laboratory Medicine

Target Cells/Metarubricyte © 2002 MTS, University of Washington Department of Laboratory Medicine

Polychromatophilic/Diffusely Basophilic RBC © 2002 MTS, University of Washington Department of Laboratory Medicine

Malarial Gametocytes © 2002 MTS, University of Washington Department of Laboratory Medicine

Hgb C Crystals © 2002 MTS, University of Washington Department of Laboratory Medicine

Retic and Heinz Bodies © 2002 MTS, University of Washington Department of Laboratory Medicine