Different Types of Formulas Molecular Formula shows of

- Slides: 13

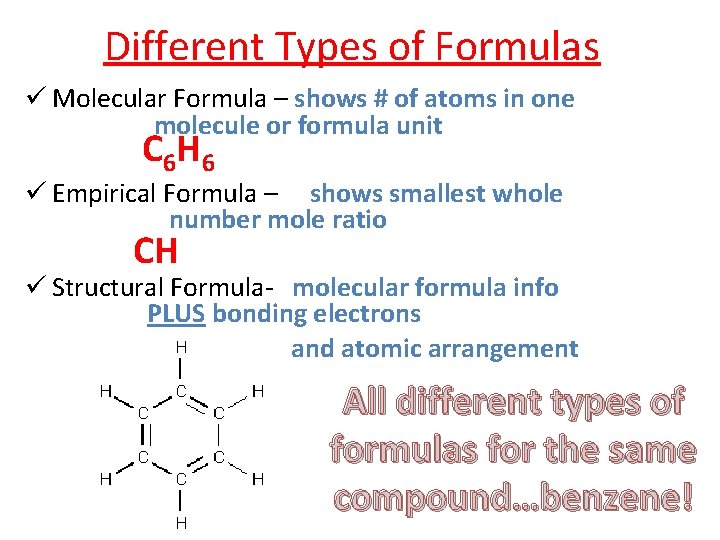
Different Types of Formulas Molecular Formula – shows # of atoms in one molecule or formula unit C 6 H 6 Empirical Formula – shows smallest whole number mole ratio CH Structural Formula- molecular formula info PLUS bonding electrons and atomic arrangement All different types of formulas for the same compound…benzene!

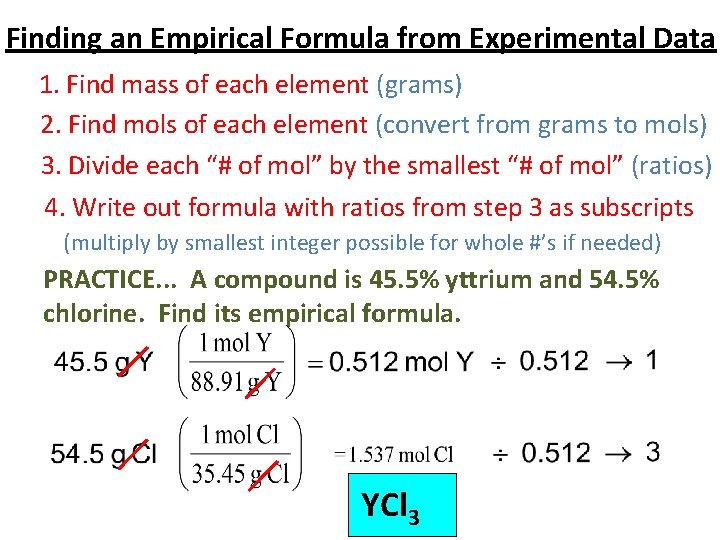
Finding an Empirical Formula from Experimental Data 1. Find mass of each element (grams) 2. Find mols of each element (convert from grams to mols) 3. Divide each “# of mol” by the smallest “# of mol” (ratios) 4. Write out formula with ratios from step 3 as subscripts (multiply by smallest integer possible for whole #’s if needed) PRACTICE. . . A compound is 45. 5% yttrium and 54. 5% chlorine. Find its empirical formula. YCl 3

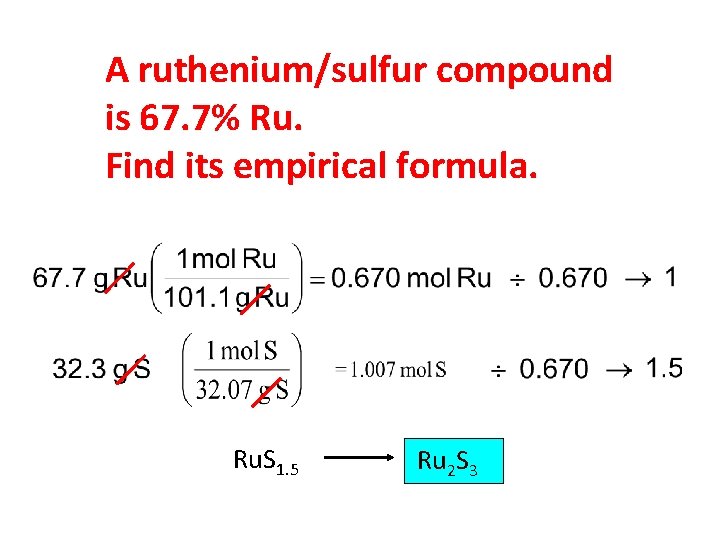
A ruthenium/sulfur compound is 67. 7% Ru. Find its empirical formula. Ru. S 1. 5 Ru 2 S 3

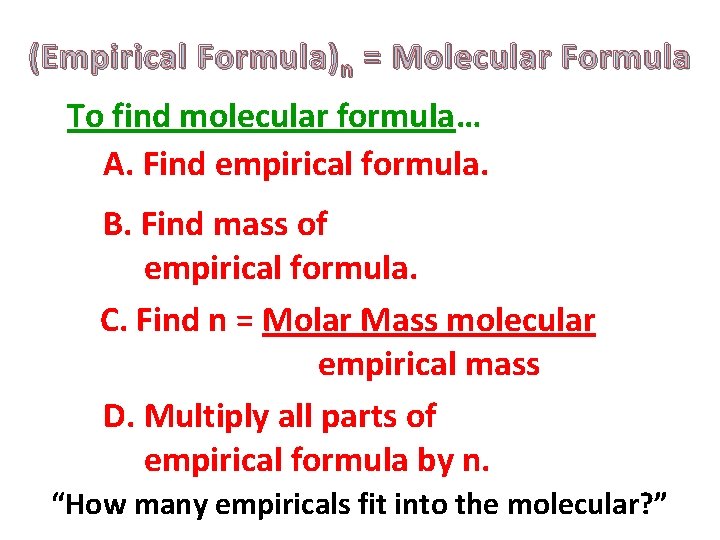
(Empirical Formula) n = Molecular Formula To find molecular formula… A. Find empirical formula. B. Find mass of empirical formula. C. Find n = Molar Mass molecular empirical mass D. Multiply all parts of empirical formula by n. “How many empiricals fit into the molecular? ”

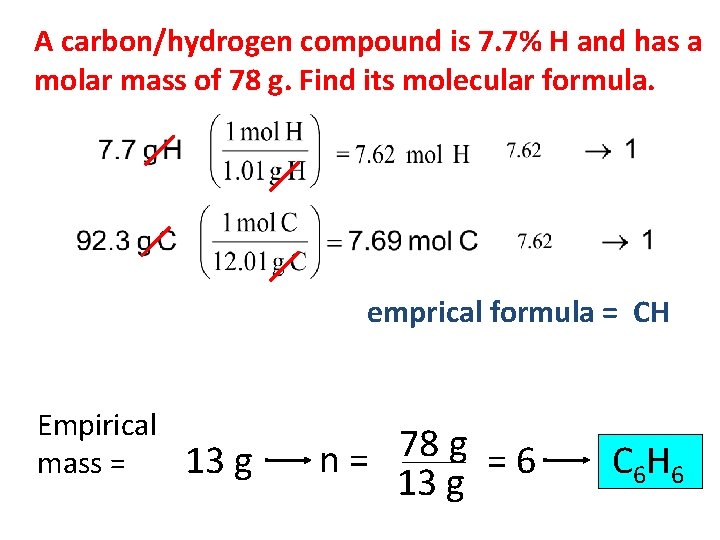
A carbon/hydrogen compound is 7. 7% H and has a molar mass of 78 g. Find its molecular formula. emprical formula = CH Empirical mass = 13 g 78 g n= =6 13 g C 6 H 6

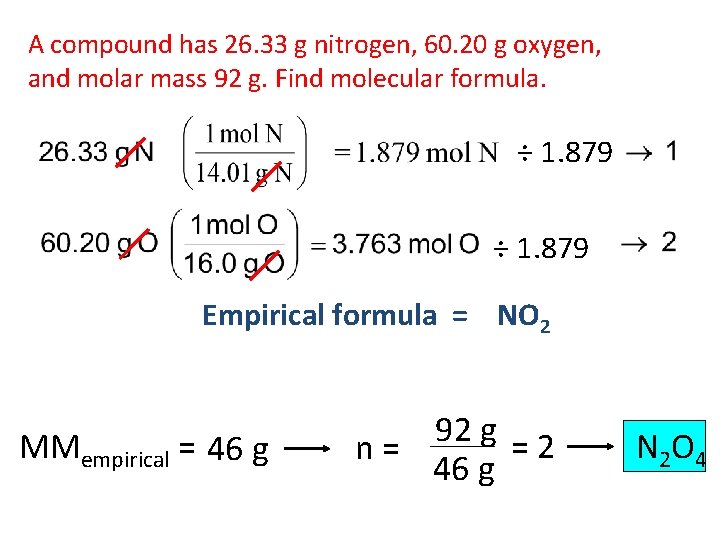
A compound has 26. 33 g nitrogen, 60. 20 g oxygen, and molar mass 92 g. Find molecular formula. ÷ 1. 879 Empirical formula = NO 2 MMempirical = 46 g 92 g = 2 n= 46 g N 2 O 4

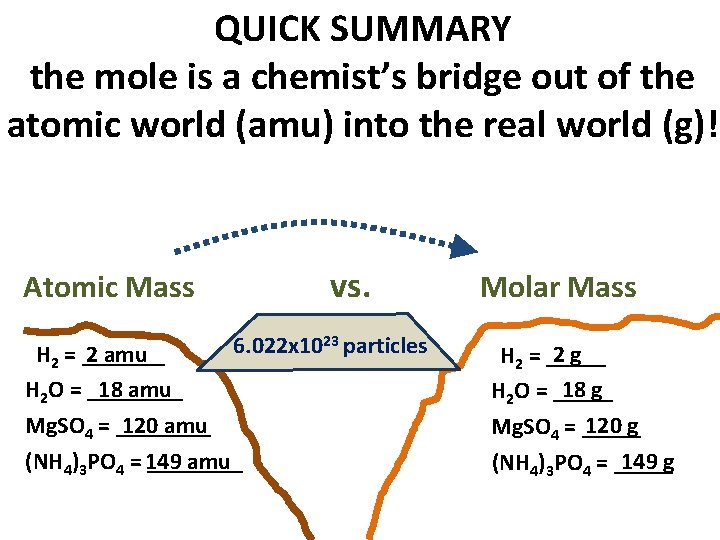
QUICK SUMMARY the mole is a chemist’s bridge out of the atomic world (amu) into the real world (g)! vs. Atomic Mass 6. 022 x 1023 particles H 2 = _______ 2 amu H 2 O = ____ 18 amu Mg. SO 4 = ____ 120 amu (NH 4)3 PO 4 = 149 ____ amu Molar Mass 2 g H 2 = _____ 18 g H 2 O = _____ 120 g Mg. SO 4 = _____ 149 g (NH 4)3 PO 4 = _____

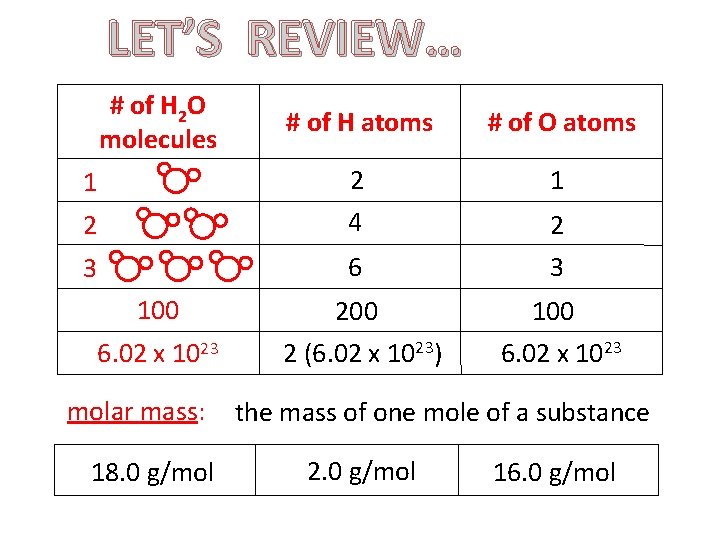
LET’S REVIEW… # of H 2 O molecules # of H atoms # of O atoms 1 2 4 2 3 6 3 100 200 100 6. 02 x 1023 2 (6. 02 x 1023) molar mass: 18. 0 g/mol 6. 02 x 1023 the mass of one mole of a substance 2. 0 g/mol 16. 0 g/mol

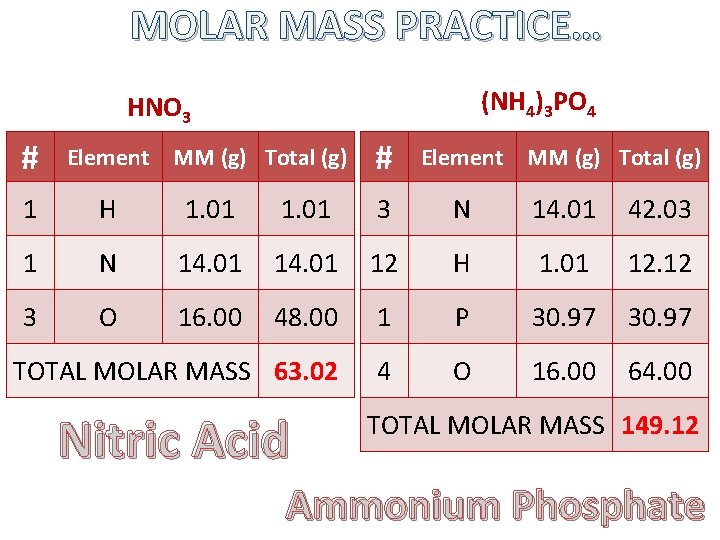
MOLAR MASS PRACTICE… (NH 4)3 PO 4 HNO 3 # Element MM (g) Total (g) 1 H 1. 01 3 N 14. 01 42. 03 1 N 14. 01 12 H 1. 01 12. 12 3 O 16. 00 48. 00 1 P 30. 97 TOTAL MOLAR MASS 63. 02 4 O 16. 00 64. 00 Nitric Acid TOTAL MOLAR MASS 149. 12 Ammonium Phosphate

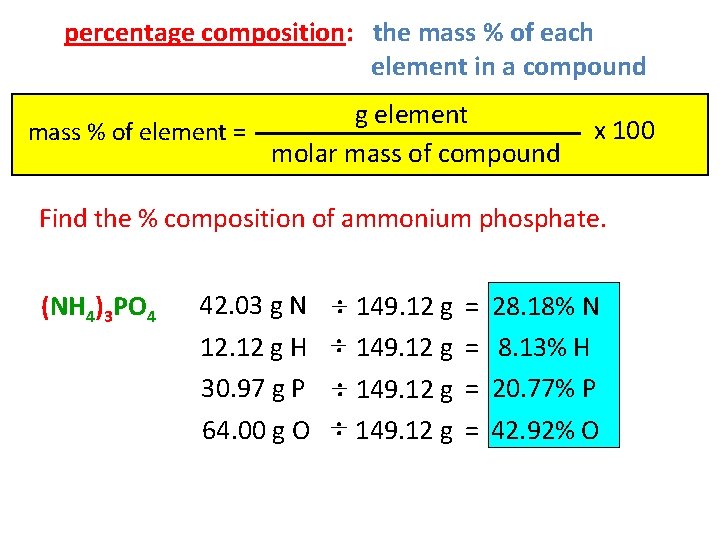
percentage composition: the mass % of each element in a compound g element mass % of element = molar mass of compound x 100 Find the % composition of ammonium phosphate. (NH 4)3 PO 4 42. 03 g N 12. 12 g H 30. 97 g P 64. 00 g O : 149. 12 g = = 28. 18% N 8. 13% H 20. 77% P 42. 92% O

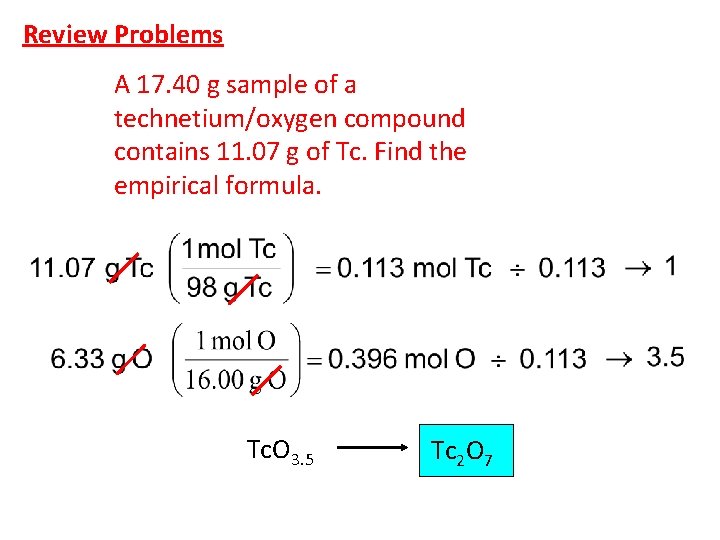
Review Problems A 17. 40 g sample of a technetium/oxygen compound contains 11. 07 g of Tc. Find the empirical formula. Tc. O 3. 5 Tc 2 O 7

Review Problems A compound contains 70. 35 g C and 14. 65 g H. Its molar mass is 58 g. Find its molecular formula. emp. form. C 2 H 5 MMemp = 29 g n= 58 g =2 29 g C 4 H 10

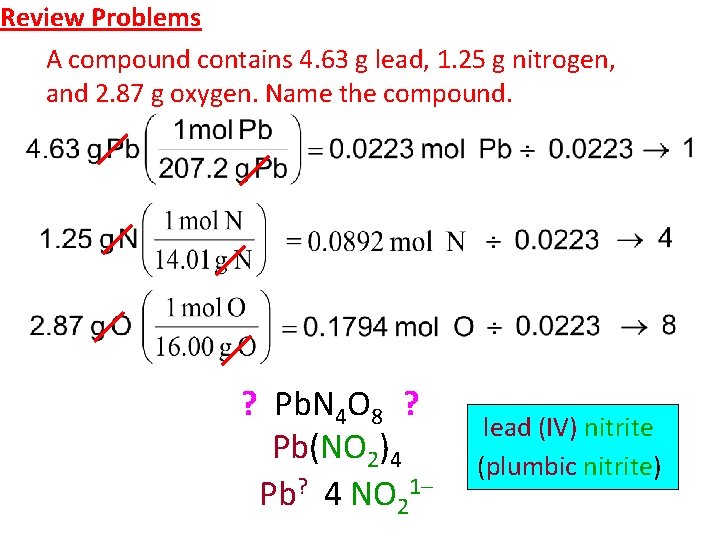
Review Problems A compound contains 4. 63 g lead, 1. 25 g nitrogen, and 2. 87 g oxygen. Name the compound. ? Pb. N 4 O 8 ? Pb(NO 2)4 Pb? 4 NO 21– lead (IV) nitrite (plumbic nitrite)