Different types of elements in the periodic table
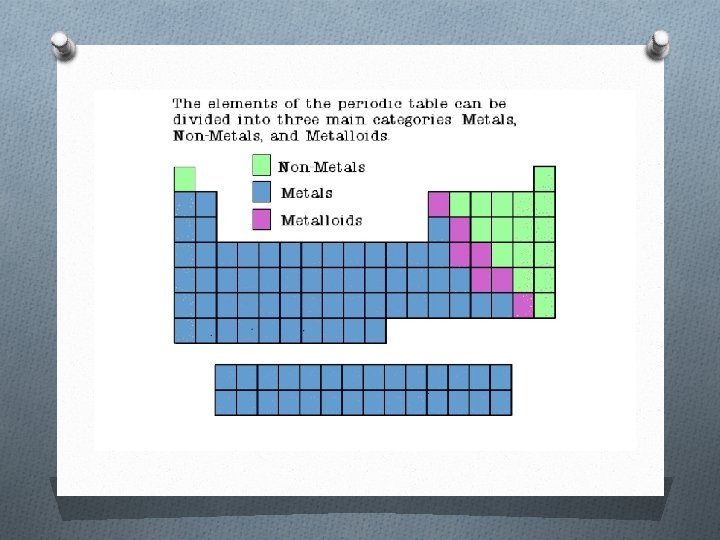
Different types of elements in the periodic table O The periodic table organizes the elements in a particular way. O A great deal of information about an element can be gathered from its position in a period table. O There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals.
Properties of Metals O Metals are good O O conductors of heat and electricity. Metals are shiny Metals are ductile ( can be stretched into thin wires) Metals are malleable (can be pounded into thin sheets. A chemical property of metal is its reaction with water which results in corrosion.
Properties of Non-Metals O Non-metals are poor conductors of heat and electricity. O Non-metals are not ductile or malleable. O Solid non-metals are brittle and break easily. O They are dull. O Many non-metals are gases.

Properties of Metalloids O Metalloids (metal-like) have properties of both metals and non-metals. O They are solids that can be shiny or dull. O They conduct heat and electricity better than non-metals but not as well as metals. O They are ductile and malleable.
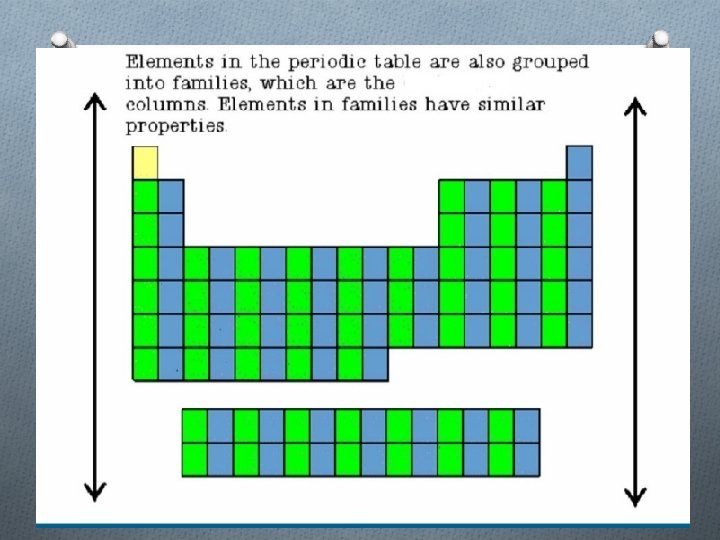
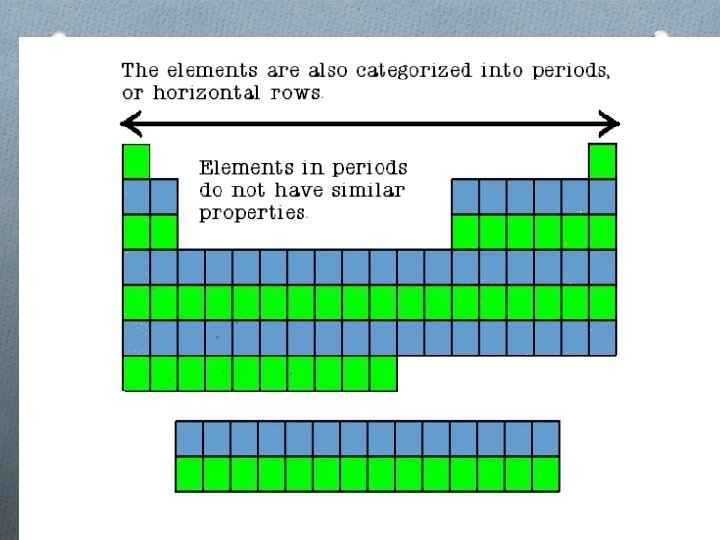

Families O Columns of elements are called groups or families O Elements in each family have similar but not identical properties. O For example, lithium (Li), sodium (Na), potassium (K), and other members of family are all soft, white, shiny metals. O All elements in a family have the same number of valence electrons. Periods O Each horizontal row of elements is called a period. O The elements in a period are not alike in properties. O In fact, the properties change greatly across even given row. O The first element in a period is always an extremely active solid. The last element in a period is always an inactive gas.

Elements Groups More specific groups are: O Alkali metals O Alkaline earth metal O Transition metals O Halogens O Noble gasses. O Rare earths
Alkali Metals O The Alkali family is founded in the first column in the periodic table. O atoms of the alkali metals have a single electron in their outermost level, In other words, 1 valence electron. O They are shiny, have the consistency of clay, and are easily cut with a knife.

Alkali Metals O They are the most reactive metals. O They react violently with water. O Alkali metals are never found as free elements in nature. O They are always bonded with another elements.
Alkaline Earth Metal O They are never found uncombined in nature. O They have two valence electrons. O Alkaline earth metal include nutrients like magnesium and calcium, among others.
Transition metals O Transition elements include O O those elements in the B families. These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. Less reactive harder metals Includes metals used in jewelry and construction.

Halogens O Halogens have 7 valence electrons, which explains why they are the most active non-metals. They are never found in nature. O Halogen atoms only need to gain 1 electron to fill their outermost energy level. O They react with alkali metals to form salts.
Noble Gasses • Noble gases are colorless gases that are extremely unreactive. • They are inactive because their outermost energy level is full. They have a full valence shell. • Because they do not readily combine with other elements to form compounds, the noble gases are called inert. • All the noble gases are found in small amounts in the earth’s atmosphere
Rare Earth Elements • The thirty rare earth elements are composed of the lanthanide and actinide series. • One element of the lanthanide series and most of the elements in the actinide serious are called trans-uranium. Which means synthetic or man-made.
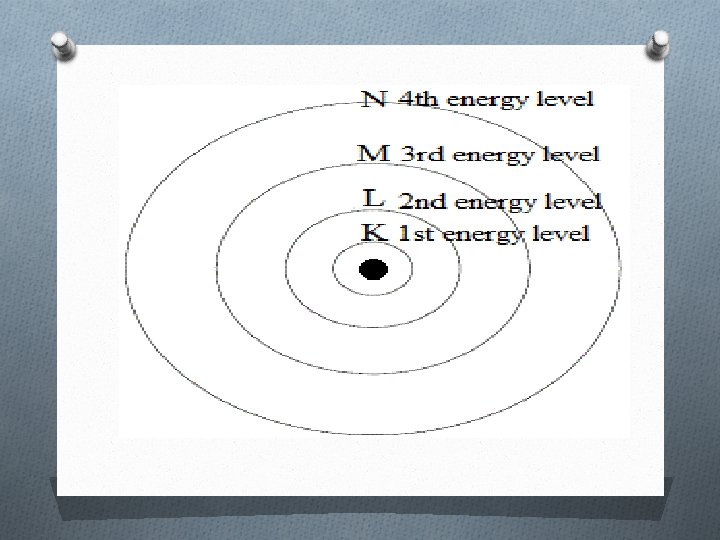
Distribution of Electrons O The distribution of the electrons in the energy shells is known as electronic configuration O Energy levels are represented by ‘ n’ values 1, 2, 3, 4 and so on.

Energy Shell O 1 st energy level is K shell: It has lowest energy. O 2 nd energy level is L shell : It has the more energy than K shells. O 3 rd energy level is M shell: It has more energy than K and L shells. O 4 th energy level is N shell: it has more energy than K , L and M shells.
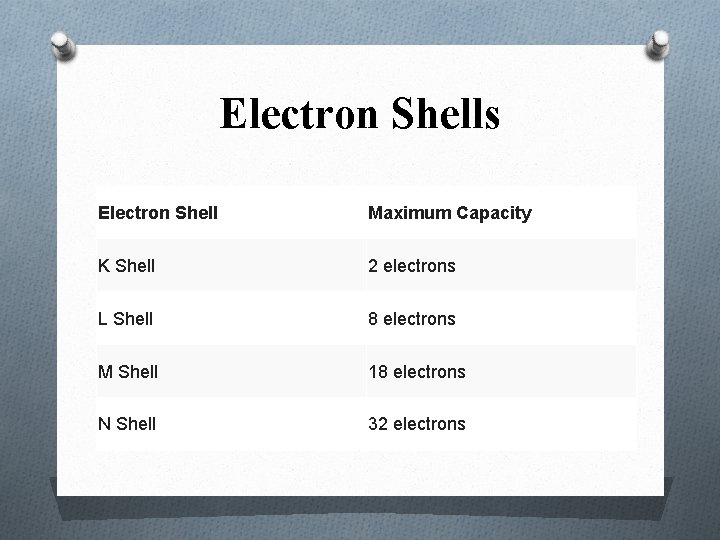
Electron Shells Electron Shell Maximum Capacity K Shell 2 electrons L Shell 8 electrons M Shell 18 electrons N Shell 32 electrons

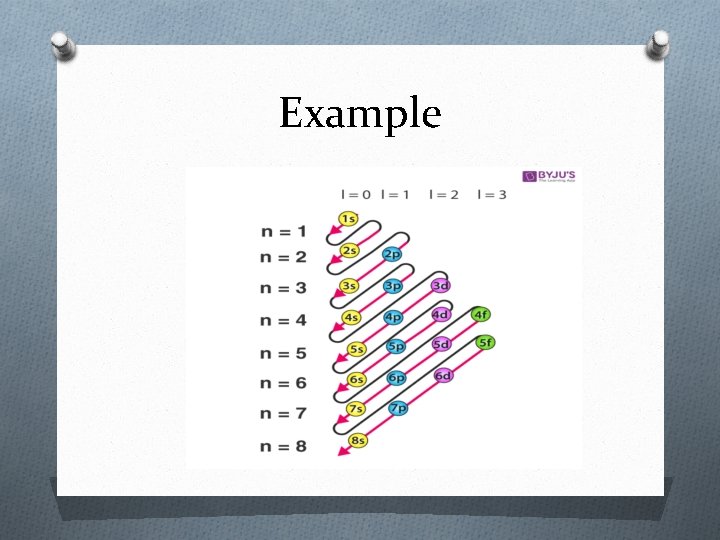
Sub shell also consists of subshells. O The number of subshell in a shell is equal to its “n” values O Each shell is designated by a small alphabetical letters s, p, d, f OA
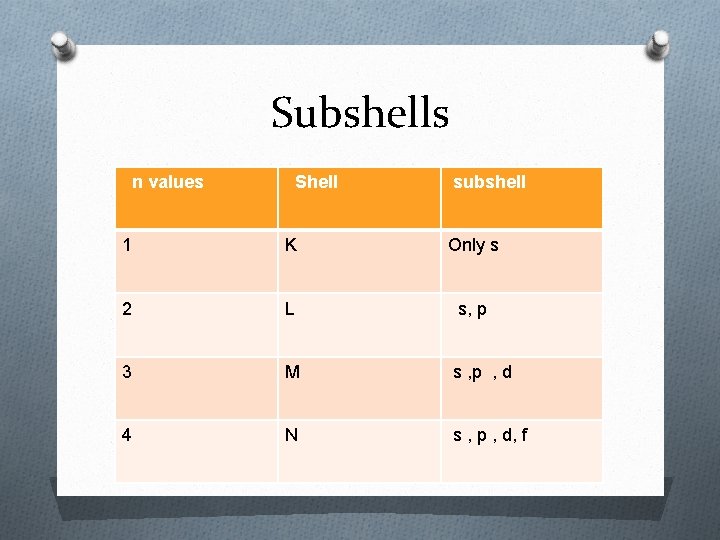
Subshells n values Shell subshell 1 K Only s 2 L s, p 3 M s , p , d 4 N s , p , d, f
Example
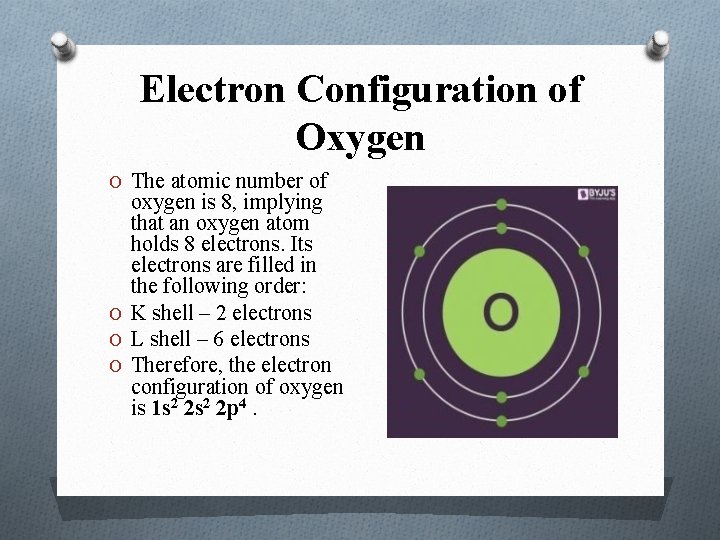
Electron Configuration of Oxygen O The atomic number of oxygen is 8, implying that an oxygen atom holds 8 electrons. Its electrons are filled in the following order: O K shell – 2 electrons O L shell – 6 electrons O Therefore, the electron configuration of oxygen is 1 s 2 2 p 4.
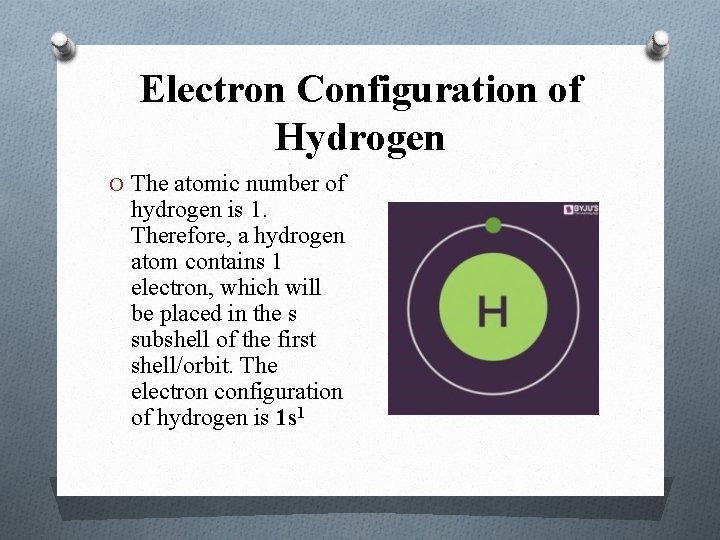
Electron Configuration of Hydrogen O The atomic number of hydrogen is 1. Therefore, a hydrogen atom contains 1 electron, which will be placed in the s subshell of the first shell/orbit. The electron configuration of hydrogen is 1 s 1
Arrangements of elements in periodic table? Definition of periodic law: n Atomic number instead of atomic mass should determine the position of elements in periodic table and accordingly the periodic law was amended as "properties of the elements are periodic function of their atomic numbers" n Atomic number of an element is equal to the number of electrons in a neutral atom. So atomic number provide the basics of electrons configurations as well.
Modern periodic table arrangements of element: n "The modern periodic table based upon the arrangement of elements according to increasing atomic number" n When the elements are arranged according to increasing atomic number from left to right in a horizontal row" properties of elements were found repeating after regular intervals such that elements of similar properties and similar configurations are placed in the same group.

Arrangements of elements in periods n The horizontal rows of elements in a periodic table n n are called periods. The elements in a period have continuously increasing atomic number. Continuously changing electronic configuration along a period. As a result properties of elements in a period are continuously changing. The number of valence electrons decides the position of an element in a period. For example, elements which have 1 electron in their valence shell occupies the left most
Arrangements of elements in periods O position in the respective periods, such as alkali metals. Similarly the elements having 8 electrons in their valence shells such as noble gases always occupy the right most position in the respective periods.
Arrangements of elements in groups n The vertical columns in a periodic table are called groups. n These groups are numbered from left to right as 1 to 18. n The elements in a group do not have continuously increasing atomic numbers. Rather the atomic number of elements in a group increase with irregular gaps. n But the elements of a group have similar electronic configuration. Same number of electrons are present in the valence shell.

Arrangements of elements in groups n For example, Arrangements the first group elements have only 1 electron in their valence shells. Similarly group 2 elements have 2 electrons in their valence shells. n It is the reason elements of a group have similar properties.
- Slides: 31