Different methods for concentration expression Mole The mole




![Molarity [Molar concentration (M)]: Number of moles of solute in one liter of solution. Molarity [Molar concentration (M)]: Number of moles of solute in one liter of solution.](https://slidetodoc.com/presentation_image_h2/86be8722142efc346d9335fb6688d792/image-7.jpg)












- Slides: 28

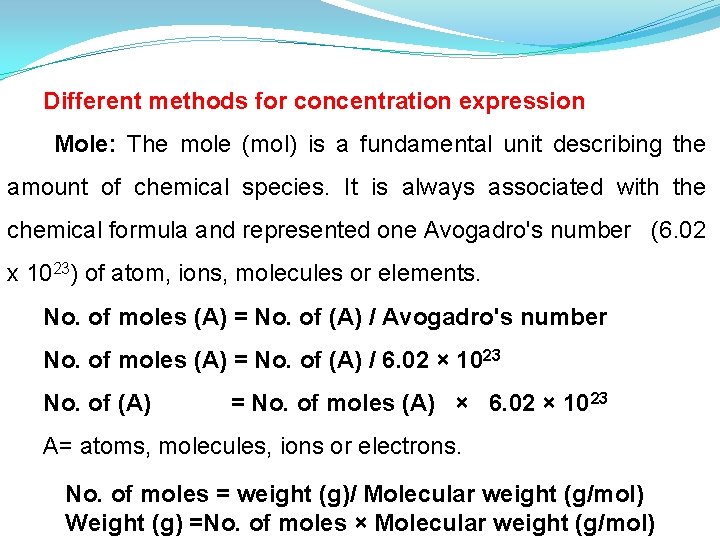
Different methods for concentration expression Mole: The mole (mol) is a fundamental unit describing the amount of chemical species. It is always associated with the chemical formula and represented one Avogadro's number (6. 02 x 1023) of atom, ions, molecules or elements. No. of moles (A) = No. of (A) / Avogadro's number No. of moles (A) = No. of (A) / 6. 02 × 1023 No. of (A) = No. of moles (A) × 6. 02 × 10 23 A= atoms, molecules, ions or electrons. No. of moles = weight (g)/ Molecular weight (g/mol) Weight (g) =No. of moles × Molecular weight (g/mol)

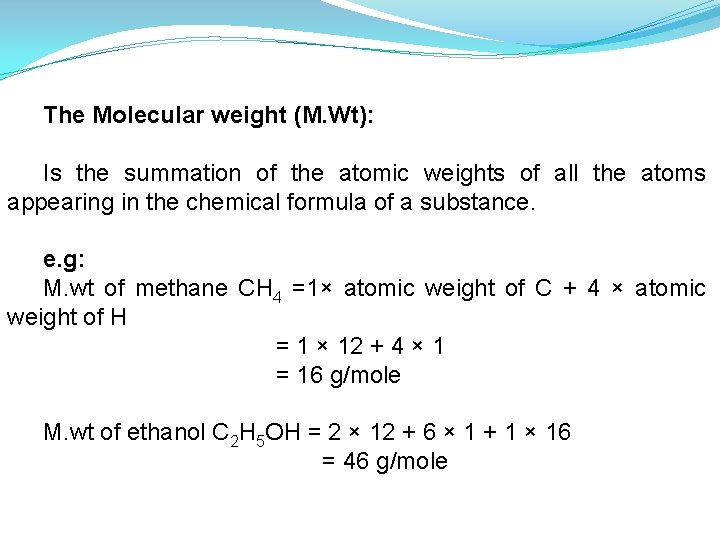
The Molecular weight (M. Wt): Is the summation of the atomic weights of all the atoms appearing in the chemical formula of a substance. e. g: M. wt of methane CH 4 =1× atomic weight of C + 4 × atomic weight of H = 1 × 12 + 4 × 1 = 16 g/mole M. wt of ethanol C 2 H 5 OH = 2 × 12 + 6 × 1 + 1 × 16 = 46 g/mole

Millimole (mmol): Some times it is more convenient to make calculations with millimoles rather than moles. 1 mol = 1000 mmol or 1 mmol = 1/1000 mol = 10 -3 mol No. of mmol = (weight (gm) / M. Wt. ) x 1000

Ex 1: How many Na+ ions are contained in 5. 0 moles of sodium ion?

Ex 2: How many moles of glucose (C 6 H 12 O 6) are contained in 36 gm of the pure sugar? If you know that: Atomic weight (At. wt): [H=1, C=12, O=16] g/mol

Ex 3: How many millimoles are contained in a) 6. 75 g of Al 2 O 3? b) 232 mg of Na 2 SO 4?
![Molarity Molar concentration M Number of moles of solute in one liter of solution Molarity [Molar concentration (M)]: Number of moles of solute in one liter of solution.](https://slidetodoc.com/presentation_image_h2/86be8722142efc346d9335fb6688d792/image-7.jpg)
Molarity [Molar concentration (M)]: Number of moles of solute in one liter of solution. M = No. of moles / volume(L) = No. of mmoles/volume (ml) No. of moles = M × V (L) or No. of mmoles = M × V (ml) No. of moles = Wt. of solute (g) /M. Wt (g/mole)

Ex : Calculate the molarity of a species in a) An aqueous solution that contains 2. 3 g of ethanol (C 2 H 5 OH) in 2. 00 L? b) An aqueous solution that contains 285 mg of trichloroacetic acid (Cl 3 CCOOH) in 10 ml? Atomic weight (At. wt): [H=1, C=12, Cl=35. 5, O=16] g/mole

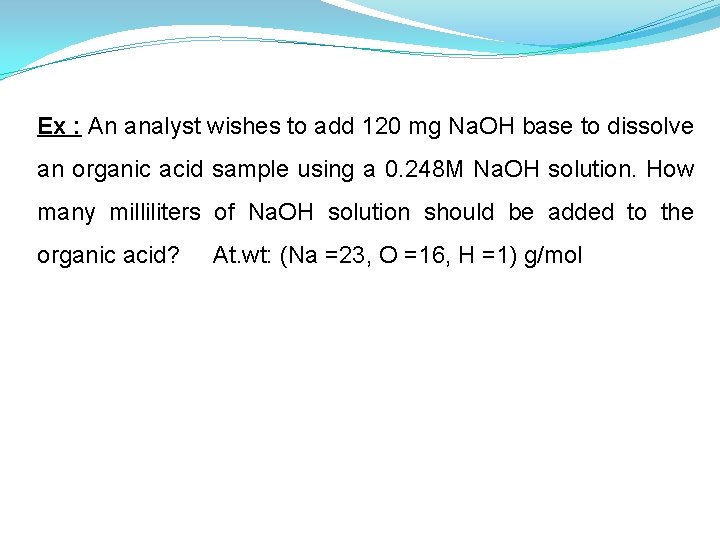
Ex : An analyst wishes to add 120 mg Na. OH base to dissolve an organic acid sample using a 0. 248 M Na. OH solution. How many milliliters of Na. OH solution should be added to the organic acid? At. wt: (Na =23, O =16, H =1) g/mol

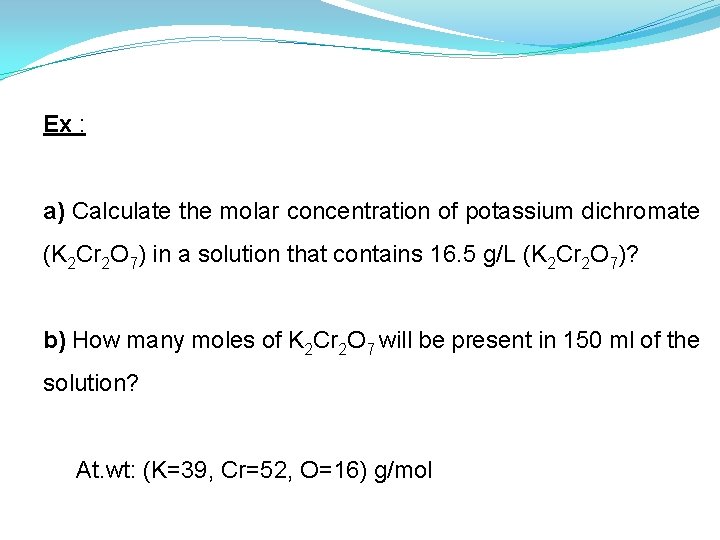
Ex : a) Calculate the molar concentration of potassium dichromate (K 2 Cr 2 O 7) in a solution that contains 16. 5 g/L (K 2 Cr 2 O 7)? b) How many moles of K 2 Cr 2 O 7 will be present in 150 ml of the solution? At. wt: (K=39, Cr=52, O=16) g/mol

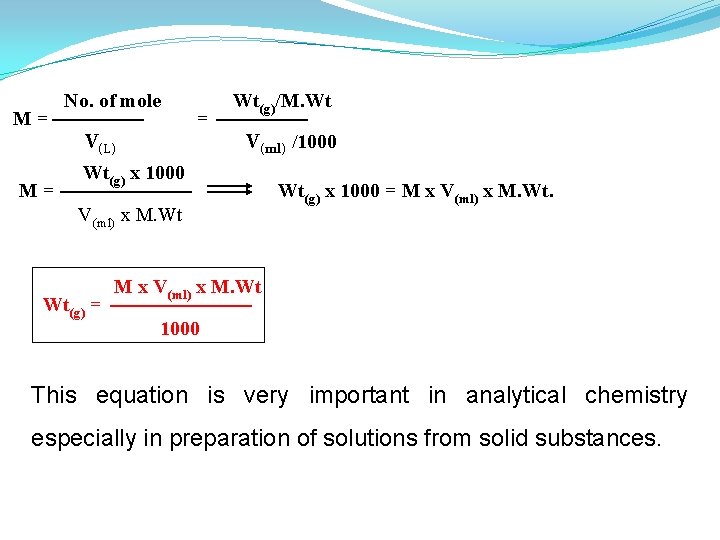
M= M= No. of mole V(L) = Wt(g)/M. Wt V(ml) /1000 Wt(g) x 1000 V(ml) x M. Wt Wt(g) = Wt(g) x 1000 = M x V(ml) x M. Wt 1000 This equation is very important in analytical chemistry especially in preparation of solutions from solid substances.

1 - Preparation of solutions from Solid compounds. Ex: Prepare 2 liter of 0. 1 M Na 2 CO 3 from the solid material. What is needed to solve this example…. . ? • Request: how much weighing from the solid material (Na 2 CO 3)? By another words : • How much gram we needed to weighing from Na 2 CO 3 and dissolving in 2 liter of water to obtaining 0. 1 M Na 2 CO 3?

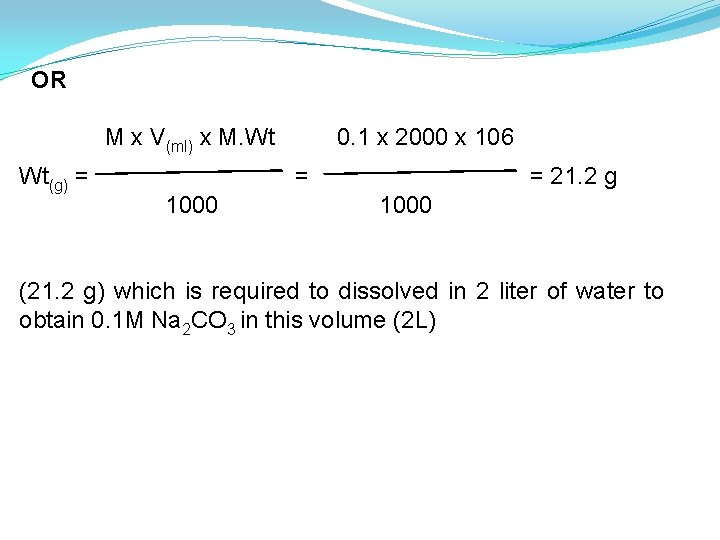
Ans. 0. 1 M = 0. 1 mole Na 2 CO 3 in one liter of solution = 0. 1 x 2 = 0. 2 mole Na 2 CO 3 in 2 liter of solution. (Because we want 2 L) Wt. (g) no. of mole = M. Wt. (g) = no. of mole x M. Wt. of Na 2 CO 3 (g) = 0. 2 x [(2 x 23) + (1 x 12) + (3 x 16)] = 21. 2 g which is required to dissolved in 2 liter of water to obtain 0. 1 M Na 2 CO 3 in this volume (2 L).

OR M x V(ml) x M. Wt Wt(g) = 0. 1 x 2000 x 106 = 1000 = 21. 2 g 1000 (21. 2 g) which is required to dissolved in 2 liter of water to obtain 0. 1 M Na 2 CO 3 in this volume (2 L)

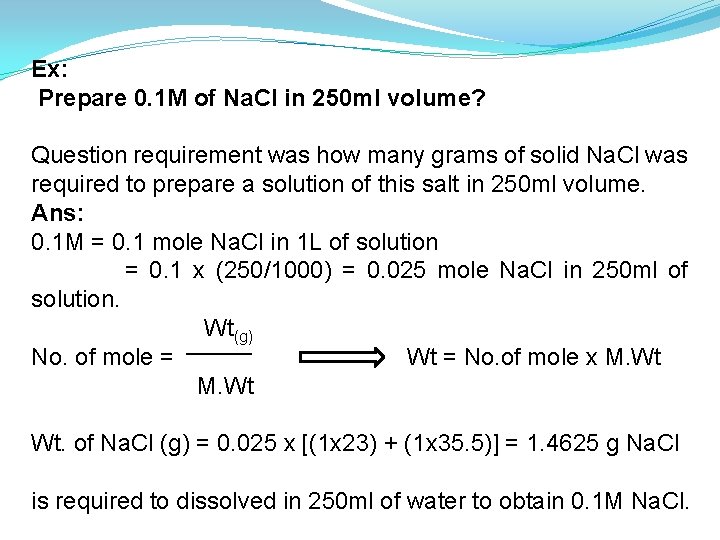
Ex: Prepare 0. 1 M of Na. Cl in 250 ml volume? Question requirement was how many grams of solid Na. Cl was required to prepare a solution of this salt in 250 ml volume. Ans: 0. 1 M = 0. 1 mole Na. Cl in 1 L of solution = 0. 1 x (250/1000) = 0. 025 mole Na. Cl in 250 ml of solution. Wt(g) No. of mole = Wt = No. of mole x M. Wt Wt. of Na. Cl (g) = 0. 025 x [(1 x 23) + (1 x 35. 5)] = 1. 4625 g Na. Cl is required to dissolved in 250 ml of water to obtain 0. 1 M Na. Cl.

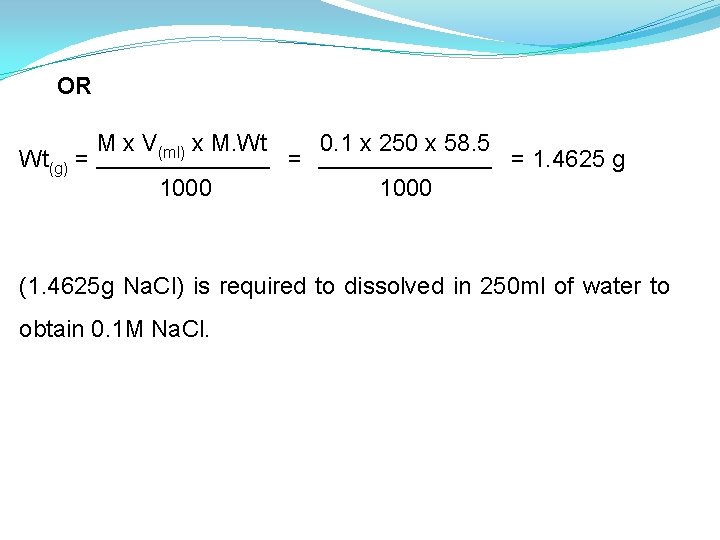
OR Wt(g) = M x V(ml) x M. Wt 1000 = 0. 1 x 250 x 58. 5 = 1. 4625 g 1000 (1. 4625 g Na. Cl) is required to dissolved in 250 ml of water to obtain 0. 1 M Na. Cl.

Q/ Explain how we can prepare each of the following solutions from pure solid substances: 1 - Prepare 0. 2 M Ba. Cl 2. 2 H 2 O in 500 ml. 2 - Prepare 0. 03 M Ca. Cl 2 in 1 L. 3 - Prepare 1 x 10 -3 M Cu. SO 4. 5 H 2 O in 100 ml. (At. Wt: Ba=137, Cl=35. 5, H=1, O=16, Ca=40, Cu=63. 5, S=32)

Ex: Prepare 0. 25 M Cl- in 250 ml from Na. Cl salt.

Ex: Prepare 2 liter of 0. 1 M Na+ from Na 2 CO 3 pure solid material. What is needed to solve this example…. . ? Request: how mush weighing (Na 2 CO 3) by grams to dissolved in 2 liter to obtain solution contained 0. 1 M Na+?

Q/ Prepare 0. 25 M Cl- in 250 ml from Ba. Cl 2. 2 H 2 O salt.

2 - Preparation of solutions from Liquids. a)Preparation of solutions from unknown concentration of liquids. This process was done by two steps: The first step must be calculated molar concentration of concentrated solution (bottle).

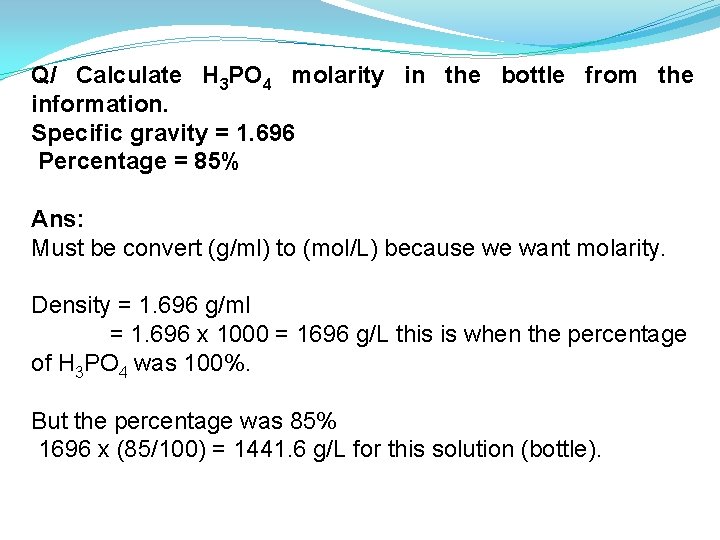
Q/ Calculate H 3 PO 4 molarity in the bottle from the information. Specific gravity = 1. 696 Percentage = 85% Ans: Must be convert (g/ml) to (mol/L) because we want molarity. Density = 1. 696 g/ml = 1. 696 x 1000 = 1696 g/L this is when the percentage of H 3 PO 4 was 100%. But the percentage was 85% 1696 x (85/100) = 1441. 6 g/L for this solution (bottle).

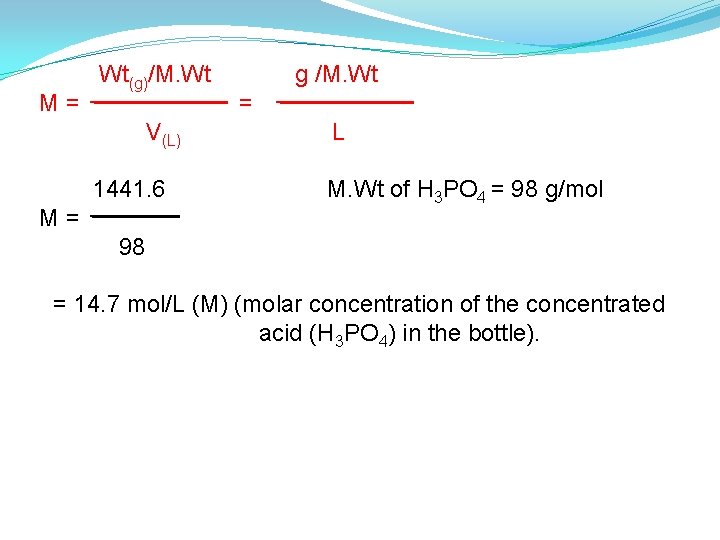
M= Wt(g)/M. Wt V(L) 1441. 6 M= g /M. Wt = L M. Wt of H 3 PO 4 = 98 g/mol 98 = 14. 7 mol/L (M) (molar concentration of the concentrated acid (H 3 PO 4) in the bottle).

There is another way to solve this example (fast way): Sp. gr. x % x 1000 M= M. Wt. For the above example…. . 1. 696 x (85/100) x 1000 M= 98 = 14. 7 mol/L (M) the same result.

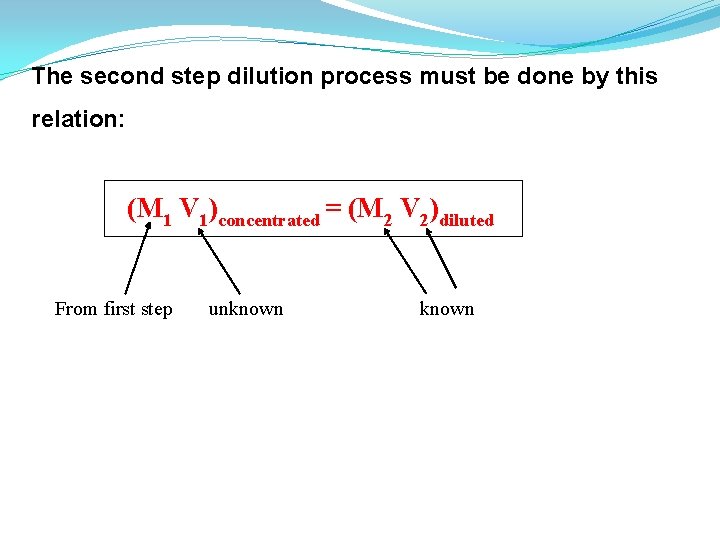
The second step dilution process must be done by this relation: (M 1 V 1)concentrated = (M 2 V 2)diluted From first step unknown

Ex: Prepare 500 ml (6 M) from the concentrated H 3 PO 4. Sp. gr. = 1. 696 , percentage = %85 , At. Wt: P=31 , O=16. Ans: The first step must be calculated molar concentration of concentrated solution (bottle).

The second step: (dilution) (M 1 V 1)concentrated = (M 2 V 2)diluted (14. 7 x V 1) = (6 x 500) V 1 = (6 x 500)/14. 7 = 204. 1 ml This is meaning we take 204. 1 ml from the concentrated solution (bottle) and diluted to 500 ml with distilled water to obtain 6 M H 3 PO 4.

Ex: Prepare 250 ml of 2 M HNO 3 from the concentrated solution. The information on the bottle: percentage = 69% , specific gravity 1. 42, M. Wt= 63 g/mole.