Differences and similarities between HACCP and Risk Analysis
Differences and similarities between HACCP and Risk Analysis Mike van Schothorst Diff Sim HA RA 1

Terminology Diff Sim HA RA 2

ALOP Communication FSO Risk Hazard MRA HACCP MRM Safety Hazard Analysis / Risk Analysis Diff Sim HA RA 3

Hazard A biological, chemical or physical agent in, or condition of, food with the potential to cause an adverse health effect Codex Alimentarius, 1997 Diff Sim HA RA 4
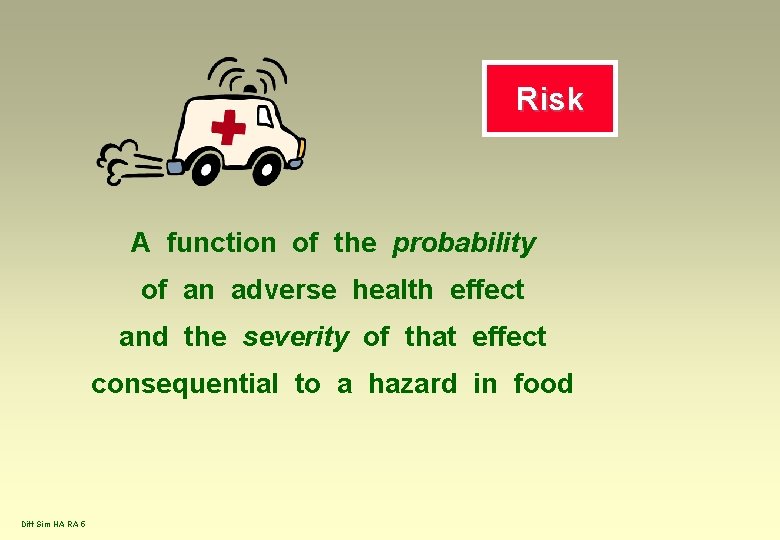
Risk A function of the probability of an adverse health effect and the severity of that effect consequential to a hazard in food Diff Sim HA RA 5

Risk Analysis A regulatory tool to maintain or enhance the supply of safe food, both locally produced and imported, in a certain country It is not only an analysis, it includes also risk management Diff Sim HA RA 6
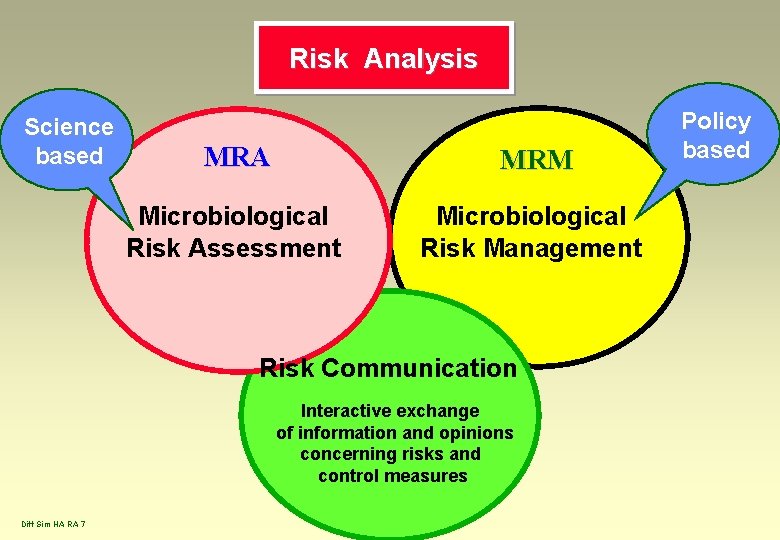
Risk Analysis Science based MRA MRM Microbiological Risk Assessment Microbiological Risk Management Risk Communication Interactive exchange of information and opinions concerning risks and control measures Diff Sim HA RA 7 Policy based
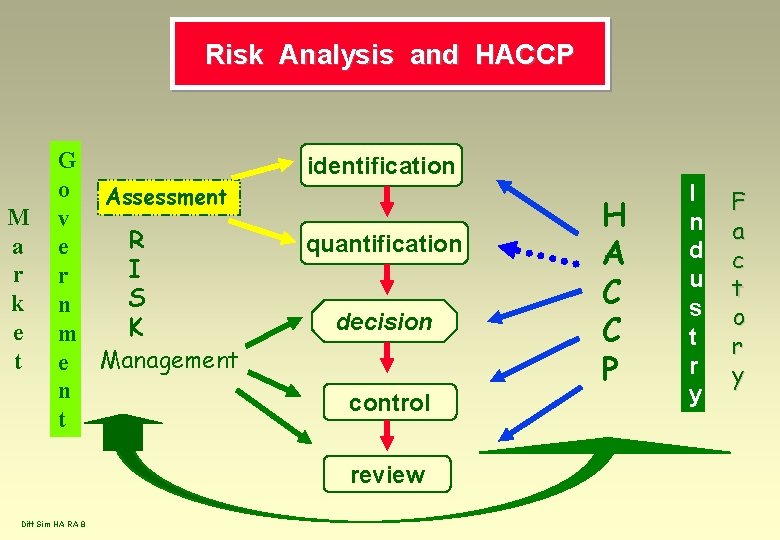
Risk Analysis and HACCP M a r k e t G o Assessment v R e I r S n K m e Management n t identification quantification decision control review Diff Sim HA RA 8 H A C C P I n d u s t r y F a c t o r y

HACCP An operational system to select and implement effective control measures to ensure the safety of a food product Microbiological Risk Assessment ( MRA ) A procedure to provide data that are used in the selection of appropriate risk reduction measures Diff Sim HA RA 9

The HACCP system compared with Risk Analysis Diff Sim HA RA 10
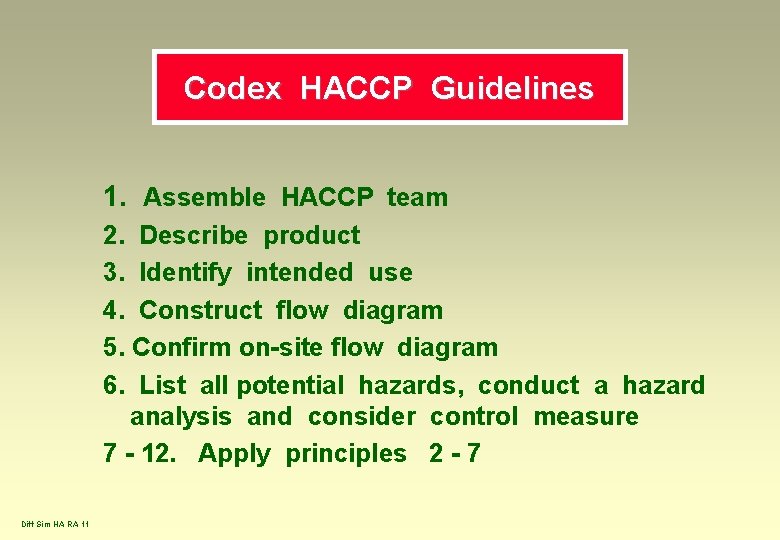
Codex HACCP Guidelines 1. Assemble HACCP team 2. Describe product 3. Identify intended use 4. Construct flow diagram 5. Confirm on-site flow diagram 6. List all potential hazards, conduct a hazard analysis and consider control measure 7 - 12. Apply principles 2 - 7 Diff Sim HA RA 11

HACCP Principles 1. 2. 3. 4. 5. 6. 7. Diff Sim HA RA 12 Conduct a hazard analysis Determine the CCPs Establish critical limit(s) Establish a monitoring system Establish corrective actions Establish verification procedures Establish documentation

(1) Assemble HACCP team Ø Obtain top management commitment Ø Appoint a leader and a secretary Ø Assure participation of experts in QA, microbiology, chemistry, food technology Ø Assure co-operation of other experts Ø Define scope of the study Ø Set priorities Diff Sim HA RA 13

Assemble Risk Assessment team Same general principles apply, but Risk managers are mainly governmental regulators and scientists, while a HACCP team consists mainly of production people. The pathogen and food of concern is often already decided upon by the Risk Managers MRA Diff Sim HA RA 14

(2) Describe product Formulation and composition Raw materials & ingredients Parameters influencing safety Processing Packaging Distribution Diff Sim HA RA 15

Products to analysed Same general principles apply, but the product is a commodity, not a specific one. It is a product produced in different manners by different manufacturers, including manufacturers in other countries MRA Diff Sim HA RA 16
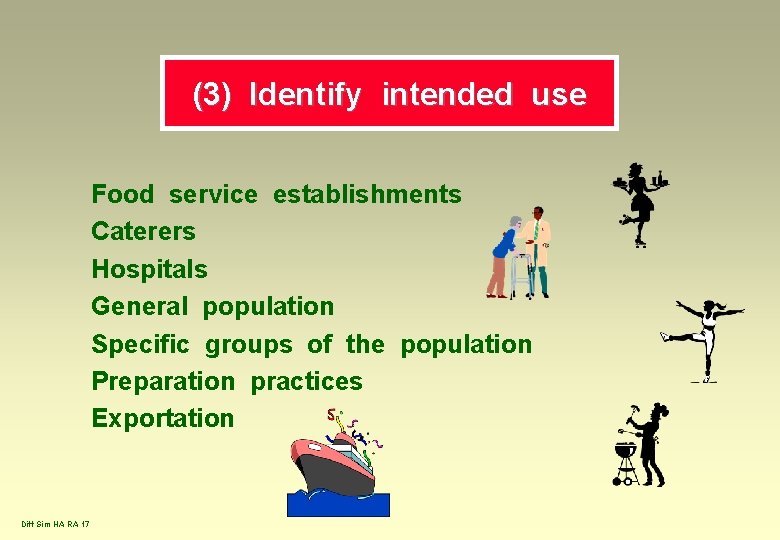
(3) Identify intended use Food service establishments Caterers Hospitals General population Specific groups of the population Preparation practices Exportation Diff Sim HA RA 17

Use of Products Same categories may apply, however, products for export may need to be treated separately because of the differences in use and users MRA Diff Sim HA RA 18
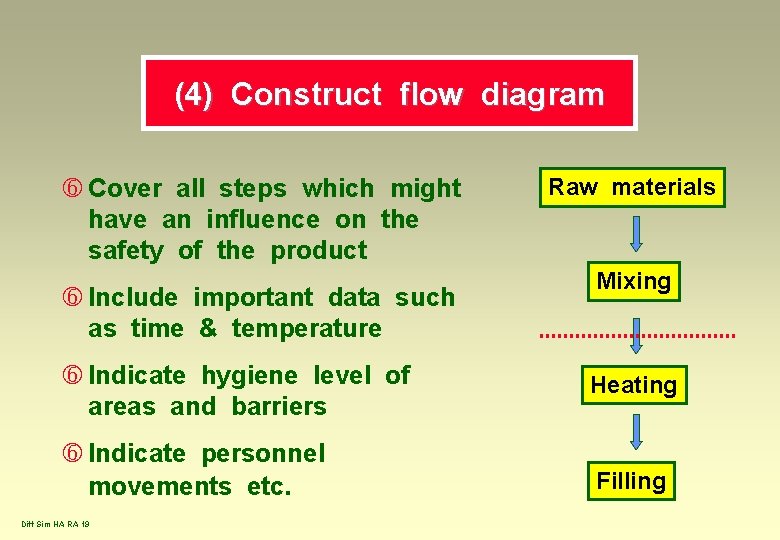
(4) Construct flow diagram Cover all steps which might have an influence on the safety of the product Include important data such as time & temperature Raw materials Mixing Indicate hygiene level of areas and barriers Heating Indicate personnel movements etc. Filling Diff Sim HA RA 19

Perform a Product / Pathogen / Pathway ( PPP ) analysis The fate of the pathogen of concern from “farm to fork” will be described in detail, data concerning conditions at the various steps need to be collected and treated, growth, survival etc. will be “modelled” MRA Diff Sim HA RA 20

(5) On - Site confirmation of flow diagram Check correctness of information Check whether important information was not overlooked Check during all periods of operation and cleaning, but also during idle hours Discuss practices with operators Diff Sim HA RA 21

PPP confirmation The pathway and its conditions need to be checked, models need to be validated and the outcomes verified as far as possible. Uncertainties need to be identified. the PPP in risk analysis is less specific as the one used in HACCP MRA Diff Sim HA RA 22

(6) List all hazards associated with each step, conduct a Hazard Analysis, consider any measures to control identified hazards Determine which potential hazards are significant and should be controled Diff Sim HA RA 23

Hazard identification The hazard of concern is identified by MRM. Important aspects of the ecology and behaviour of the pathogen are collected. This is particularly difficult when the risk of a “new” pathogen is assessed in HACCP hazard identification means determination which hazards are significant MRA Diff Sim HA RA 24

Perform a Hazard Analysis Collect and interpret information on hazards and conditions leading to their presence at unacceptable levels and decide which need to be controlled "the analysis of hazards HA is not RA must be quantitative if it is to be meaningful" ICMSF 1988 Diff Sim HA RA 25
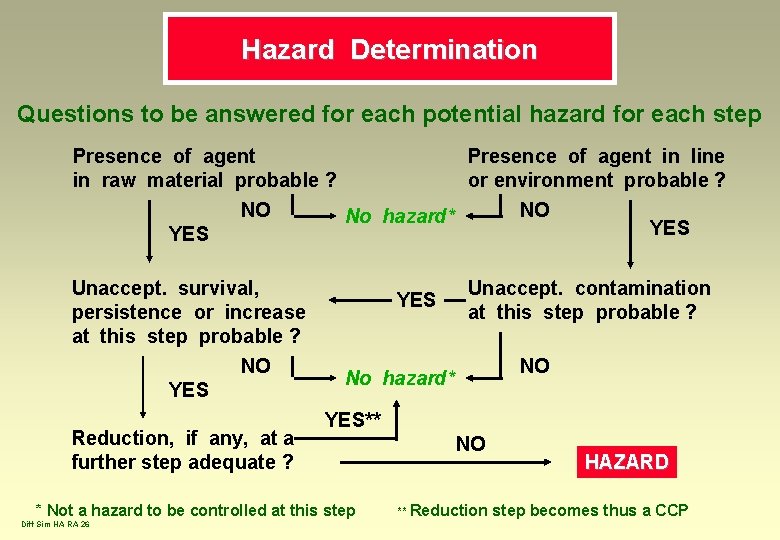
Hazard Determination Questions to be answered for each potential hazard for each step Presence of agent in line in raw material probable ? or environment probable ? NO NO No hazard* YES Unaccept. survival, persistence or increase at this step probable ? NO YES Reduction, if any, at a further step adequate ? YES NO No hazard* YES** * Not a hazard to be controlled at this step Diff Sim HA RA 26 Unaccept. contamination at this step probable ? NO ** Reduction HAZARD step becomes thus a CCP

Acceptable levels (1) Not all levels (or sizes) of all agents are harmful to all individuals under all conditions Agents (contaminants) are acceptable as long as their levels remain below a certain maximum Diff Sim HA RA 27

Acceptable levels (2) Products with a good record of safety are used as a “benchmark” New products, or changed products should be as safe as the “benchmark” Performance Objectives are “benchmarks” set by authorities Diff Sim HA RA 28

Hazard Analysis of aflatoxin in milk Maximum Level accepted 15 μg / kg Possible Probable Likely No CCP Q 1: Presence of hazard at unacceptable levels in raw material Q 2: Persistent during processing Q 3: Recontamination Q 4: Increase during shelf-life Q 5: Reduction during preparation Diff Sim HA RA 29

HA of Listeria in hotdogs Maximum Level accepted <100 / g ? Possible Probable Likely No Q 1: Presence of hazard at unacceptable levels in raw meat Q 2: Survival during processing Q 3: Recontamination Q 4: Increase during shelf-life Q 5: Reduction during preparation Diff Sim HA RA 30 CCP or
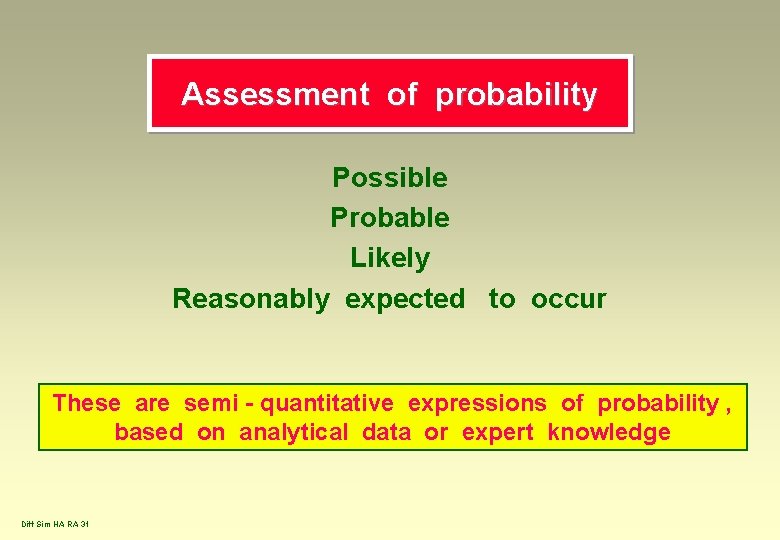
Assessment of probability Possible Probable Likely Reasonably expected to occur These are semi - quantitative expressions of probability , based on analytical data or expert knowledge Diff Sim HA RA 31

Probabilities Risk assessors use “models” to calculate probabilities of survival, persistence, growth etc. Models for recontamination are being developed the same models can be and are used in HACCP MRA Diff Sim HA RA 32

MRA components Ø Hazard identification which pathogen will be assessed Ø Hazard characterization what are the effects and what influences the effects Ø Exposure assessment how often and how many will be ingested Ø Risk characterization what is the chance that the effects will happen MRA Diff Sim HA RA 33
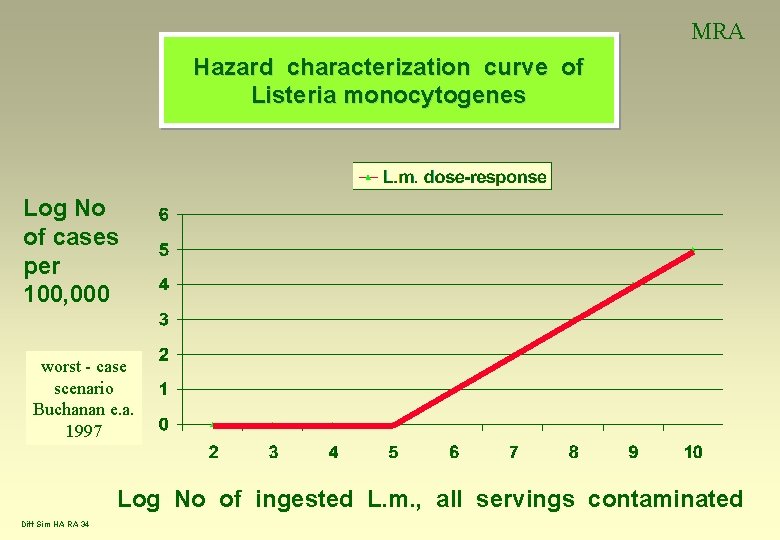
MRA Hazard characterization curve of Listeria monocytogenes Log No of cases per 100, 000 worst - case scenario Buchanan e. a. 1997 Log No of ingested L. m. , all servings contaminated Diff Sim HA RA 34
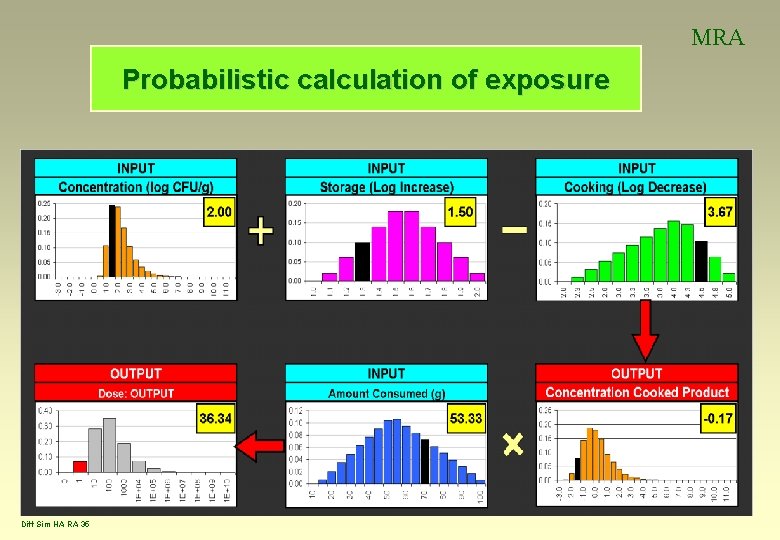
MRA Probabilistic calculation of exposure Diff Sim HA RA 35
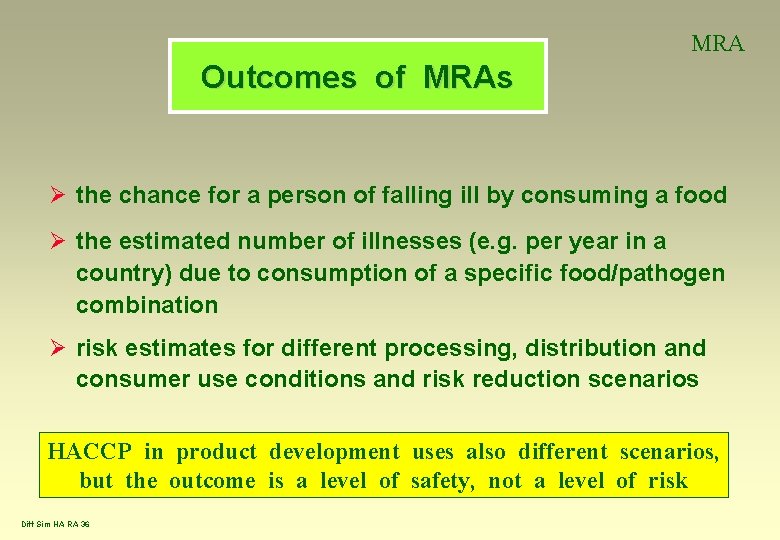
MRA Outcomes of MRAs Ø the chance for a person of falling ill by consuming a food Ø the estimated number of illnesses (e. g. per year in a country) due to consumption of a specific food/pathogen combination Ø risk estimates for different processing, distribution and consumer use conditions and risk reduction scenarios HACCP in product development uses also different scenarios, but the outcome is a level of safety, not a level of risk Diff Sim HA RA 36

7) Determine Control Measures Ø Determine where measures must be taken (CCPs) Ø Determine how and to which extent they are to be controlled at these CCPs Ø Establish the critical limits and monitoring procedures Diff Sim HA RA 37
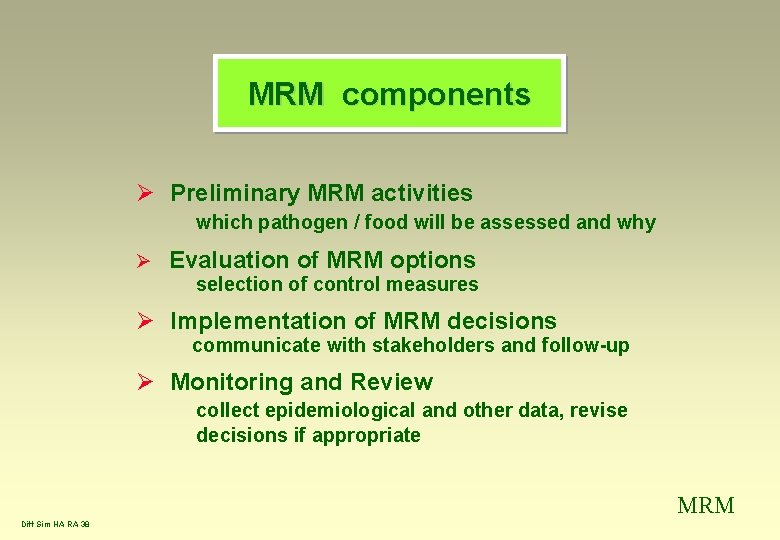
MRM components Ø Preliminary MRM activities which pathogen / food will be assessed and why Ø Evaluation of MRM options selection of control measures Ø Implementation of MRM decisions communicate with stakeholders and follow-up Ø Monitoring and Review collect epidemiological and other data, revise decisions if appropriate MRM Diff Sim HA RA 38

Control measures Risk managers are responsible for the evaluation and selection of control measures. Food business operators are responsible for their execution in HACCP both activities are in the same hands MRM Diff Sim HA RA 39

Establish Critical Limits They must assure that the required level of safety is obtained This level can be the “benchmark” or the “Performance Objective (PO)” The PO may be the outcome of MRM evaluation Diff Sim HA RA 40

MRM Performance Objective ( PO ) The maximum frequency and / or concentration of a microbial hazard in a food at a specified step in the food chain before time of consumption that still provides or contributes to the achievement of an Food Safety Objective or Appropriate Level Of Protection, as applicable. A PO is an acceptable level of a hazard in HACCP terminology Diff Sim HA RA 41
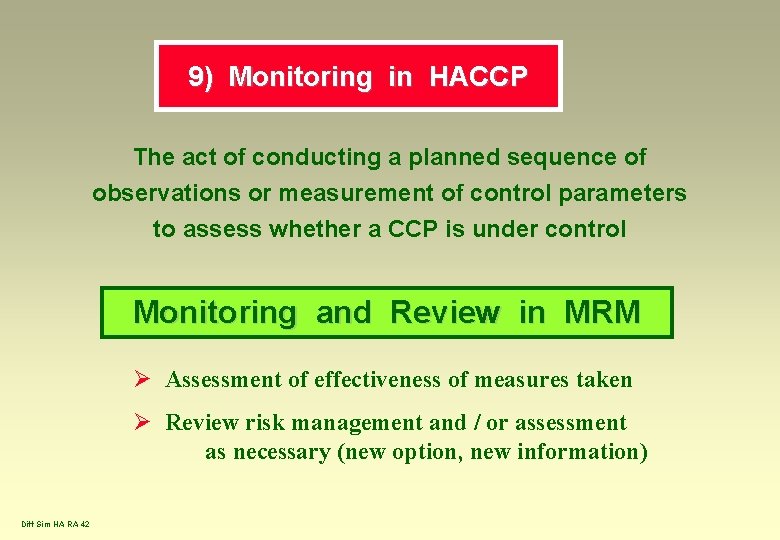
9) Monitoring in HACCP The act of conducting a planned sequence of observations or measurement of control parameters to assess whether a CCP is under control Monitoring and Review in MRM Ø Assessment of effectiveness of measures taken Ø Review risk management and / or assessment as necessary (new option, new information) Diff Sim HA RA 42

An example of differences and similarities Product Pathogen Pathway of Listeria monocytogenes in a specific paté produced according to GHP and HACCP and a “generic” paté, as used in Microbiological Risk Assessment Diff Sim HA RA 43
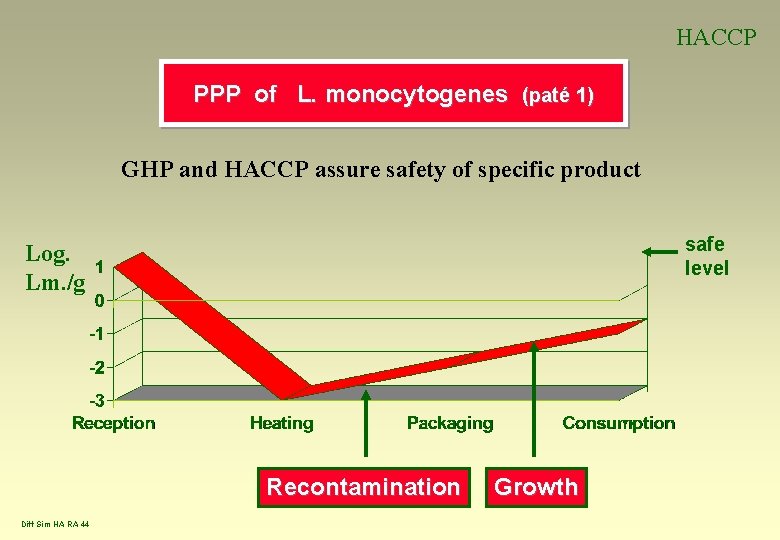
HACCP PPP of L. monocytogenes (paté 1) GHP and HACCP assure safety of specific product safe level Log. Lm. /g Recontamination Diff Sim HA RA 44 Growth
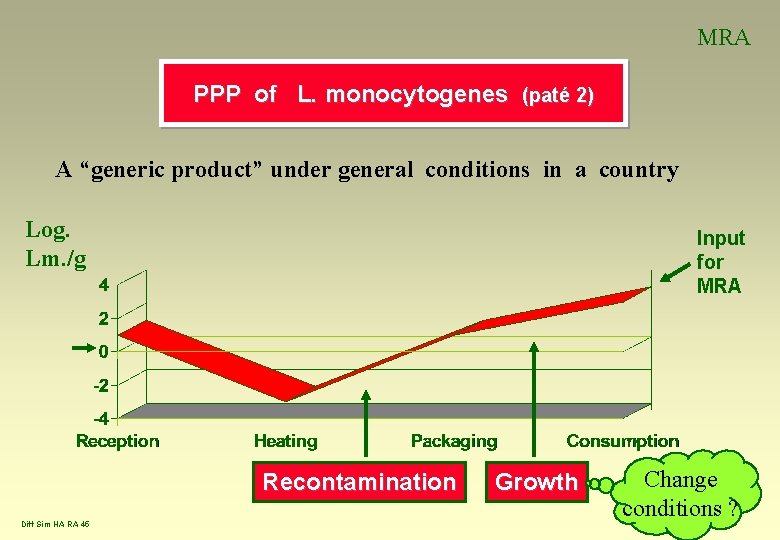
MRA PPP of L. monocytogenes (paté 2) A “generic product” under general conditions in a country Log. Lm. /g Input for MRA Recontamination Diff Sim HA RA 45 Growth Change conditions ?
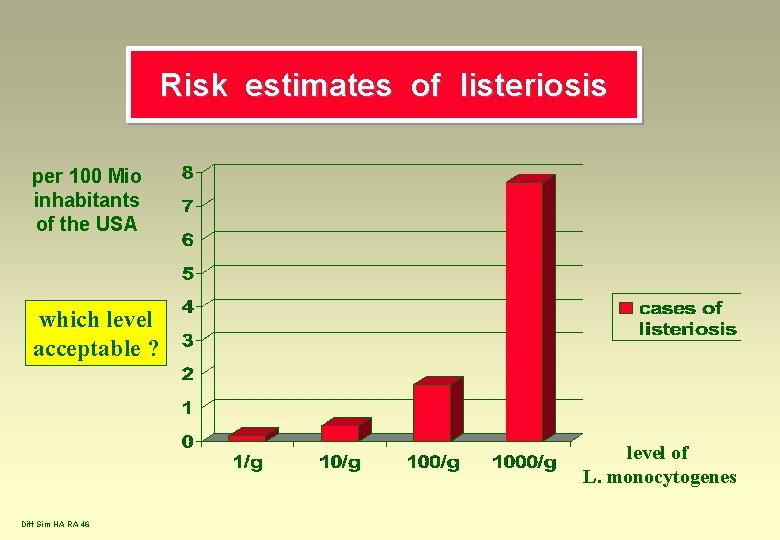
Risk estimates of listeriosis per 100 Mio inhabitants of the USA which level acceptable ? level of L. monocytogenes Diff Sim HA RA 46
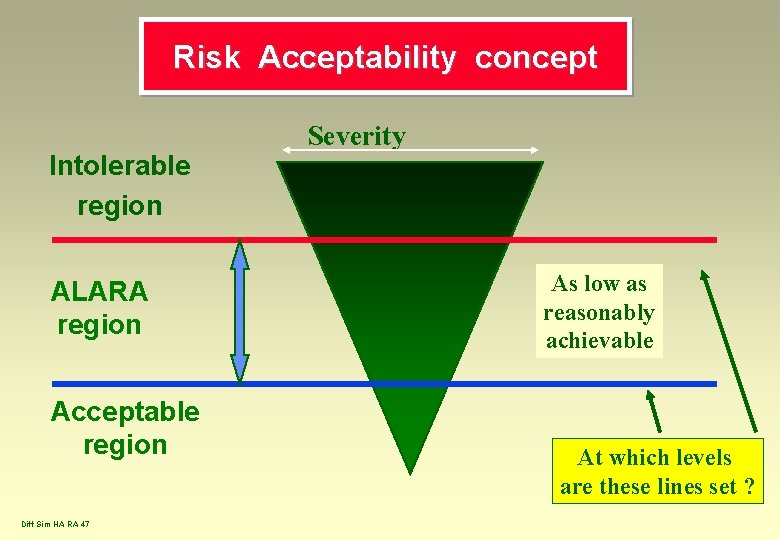
Risk Acceptability concept Intolerable region ALARA region Acceptable region Diff Sim HA RA 47 Severity As low as reasonably achievable At which levels are these lines set ?

Diff Sim HA RA 48
- Slides: 48