Diatomic elements There are 7 elements that always











- Slides: 27

Diatomic elements There are 7 elements that always form molecules: H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , I 2 The –gens (hydrogen, nitrogen, oxygen and halogens) • • NEVER FOUND AS SINGLE ATOMS Their names are just the name of the element • Oxygen by itself means O 2

Ionic Compound Formulas www. lab-initio. com

Ions ØCation: A positive ion ØMg 2+, NH 4+ ØAnion: A negative ion ØCl-, SO 42 -

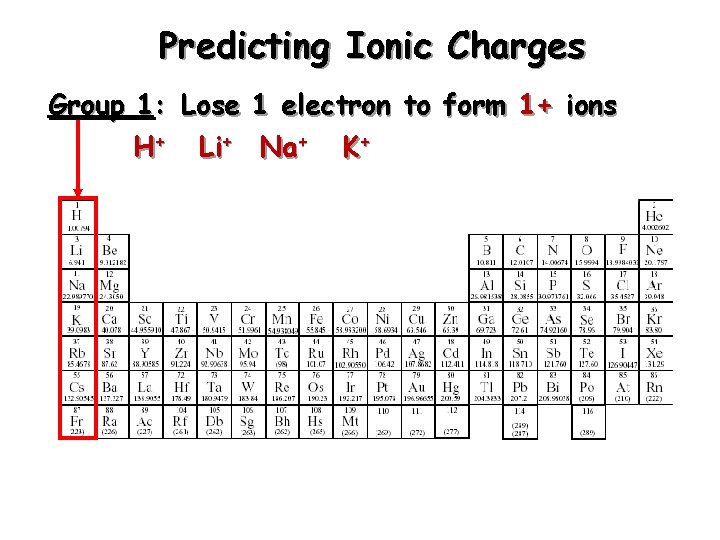
Predicting Ionic Charges Group 1: Lose 1 electron to form 1+ ions H+ Li+ Na+ K+

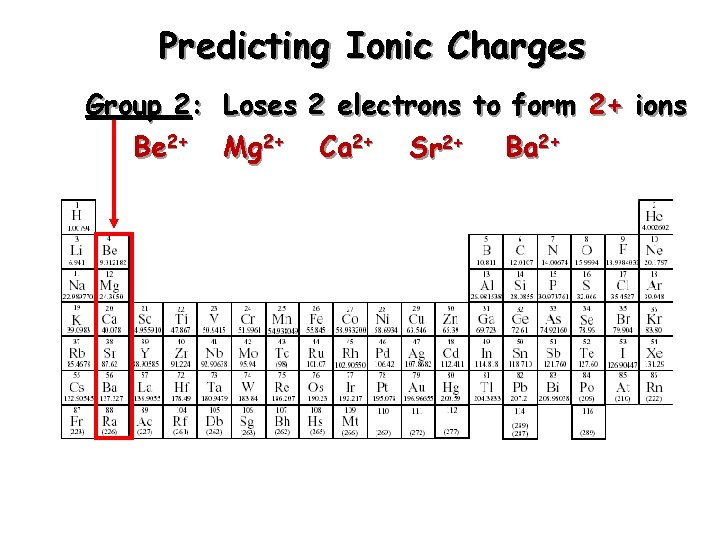
Predicting Ionic Charges Group 2: Loses 2 electrons to form 2+ ions Be 2+ Mg 2+ Ca 2+ Sr 2+ Ba 2+

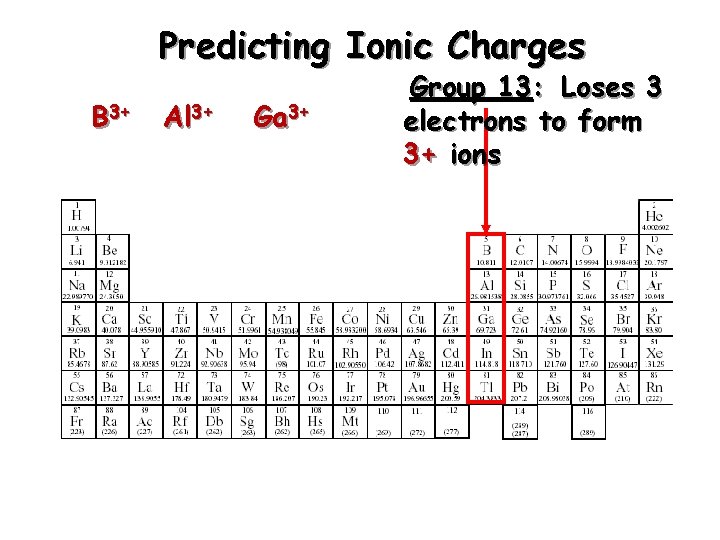
Predicting Ionic Charges B 3+ Al 3+ Ga 3+ Group 13: Loses 3 electrons to form 3+ ions

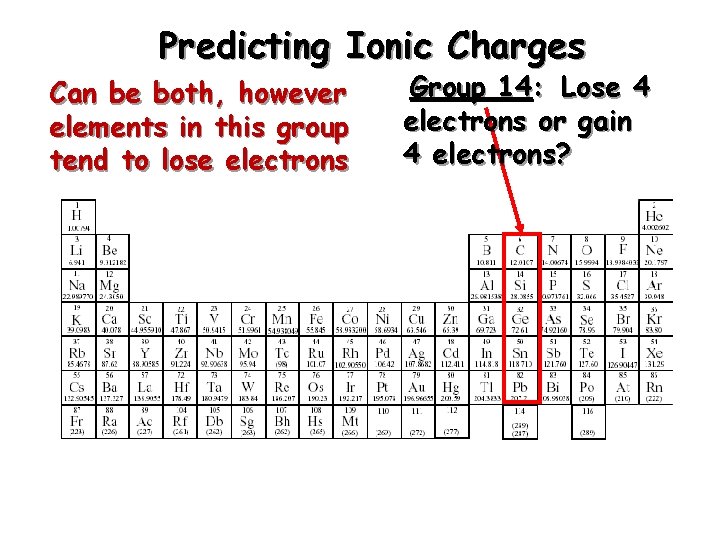
Predicting Ionic Charges Can be both, however elements in this group tend to lose electrons Group 14: Lose 4 electrons or gain 4 electrons?

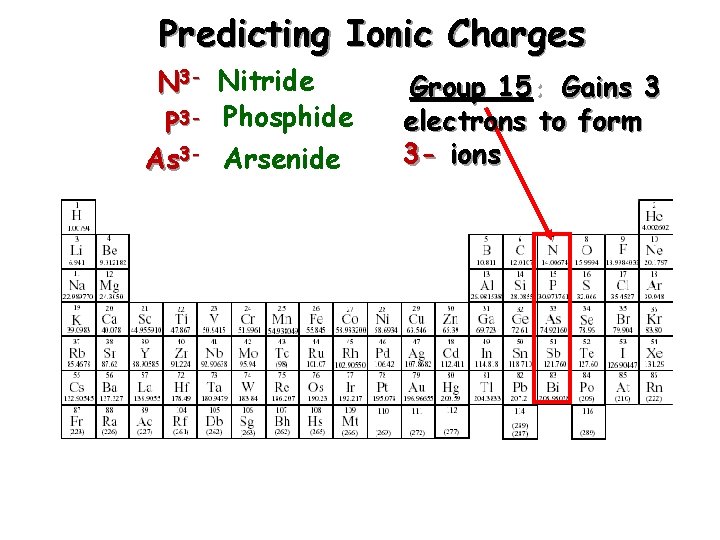
Predicting Ionic Charges N 3 - Nitride P 3 - Phosphide As 3 - Arsenide Group 15: Gains 3 electrons to form 3 - ions

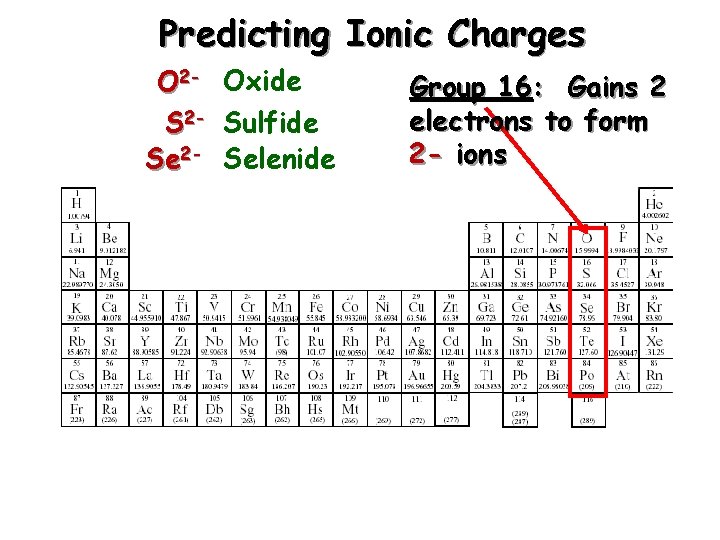
Predicting Ionic Charges O 2 - Oxide S 2 - Sulfide Se 2 - Selenide Group 16: Gains 2 electrons to form 2 - ions

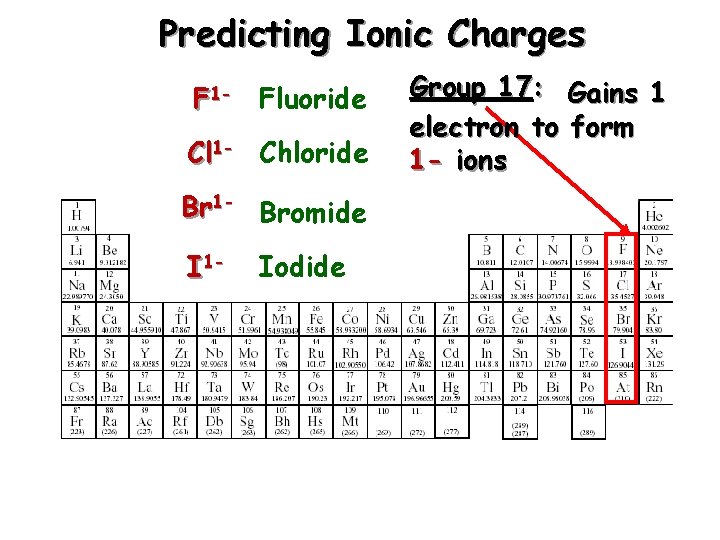
Predicting Ionic Charges F 1 - Fluoride Cl 1 - Chloride Br 1 - Bromide I 1 - Iodide Group 17: Gains 1 electron to form 1 - ions

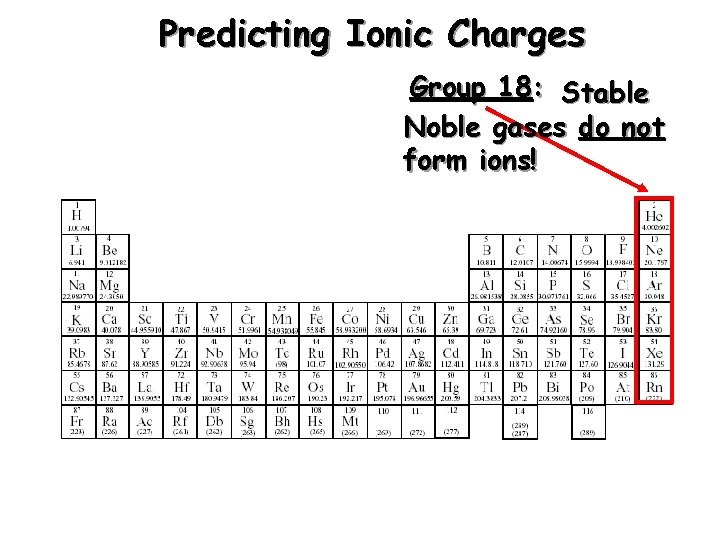
Predicting Ionic Charges Group 18: Stable Noble gases do not form ions!

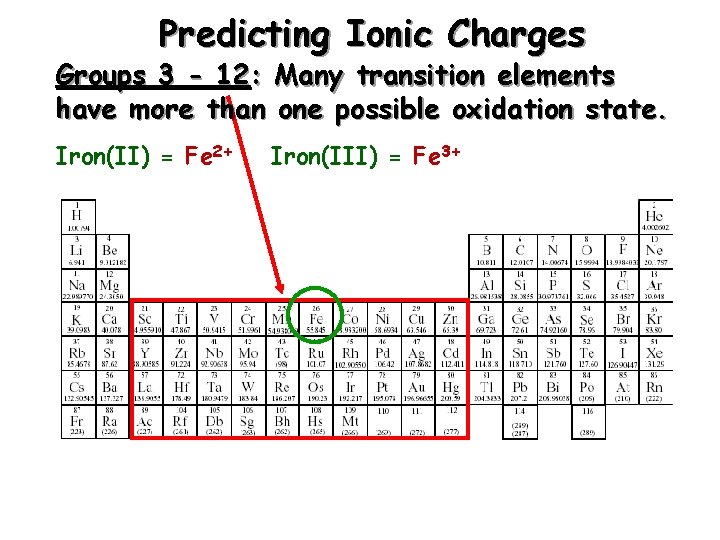
Predicting Ionic Charges Groups 3 - 12: Many transition elements have more than one possible oxidation state. Iron(II) = Fe 2+ Iron(III) = Fe 3+

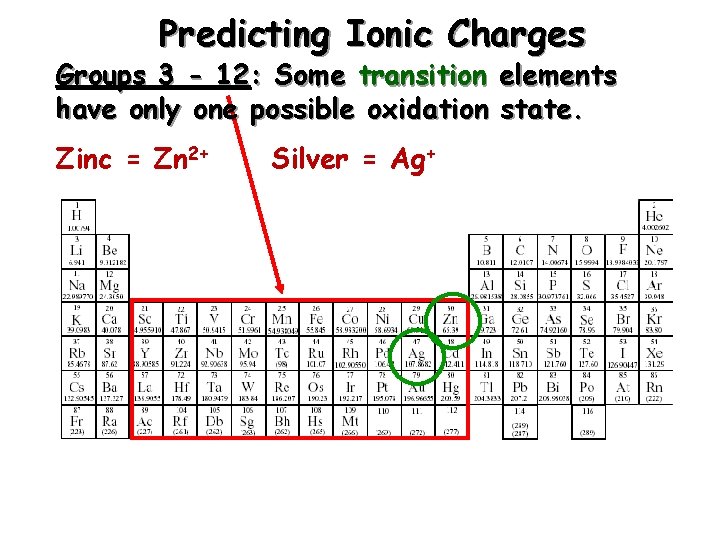
Predicting Ionic Charges Groups 3 - 12: Some transition elements have only one possible oxidation state. Zinc = Zn 2+ Silver = Ag+

Polyatomic Ions Writing Formulas / Naming Compounds • A polyatomic ion is a covalent molecule that has an ionic charge. (As opposed to being a neutral molecule. ) • Poly = many • Atomic = atoms • Ion = charged particle • A charged particle that consists of more than one atom.

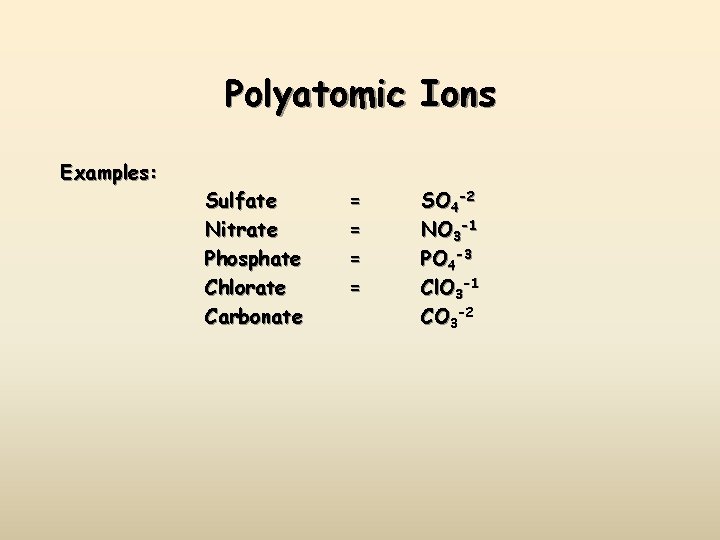
Polyatomic Ions Examples: Sulfate Nitrate Phosphate Chlorate Carbonate = = SO 4 -2 NO 3 -1 PO 4 -3 Cl. O 3 -1 CO 3 -2

Writing Formulas Sodium Sulfate • Write the formula for the metal. Add the oxidation number from the periodic table. Na+1 • Write the formula for the polyatomic ion from the ion chart. Add its oxidation number. SO 4 -2

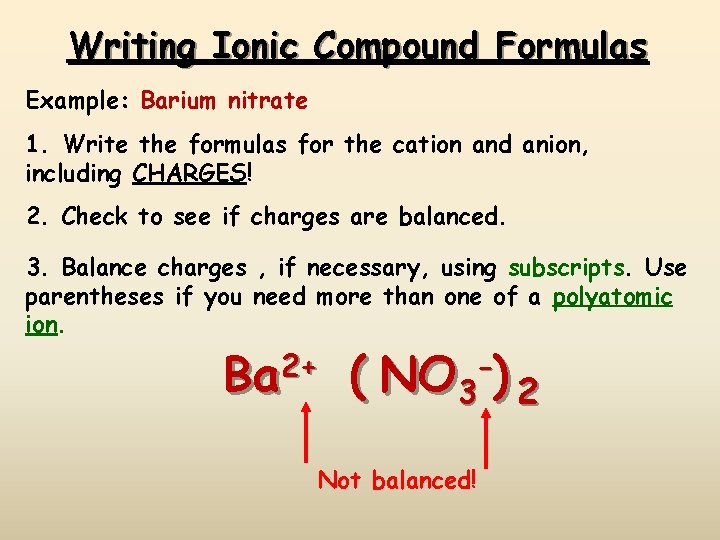
Writing Ionic Compound Formulas Example: Barium nitrate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Ba 2+ ( NO 3 -) 2 Not balanced!

Writing Ionic Compound Formulas Example: Ammonium sulfate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. + ( NH 4 ) SO 422 2 Not balanced!

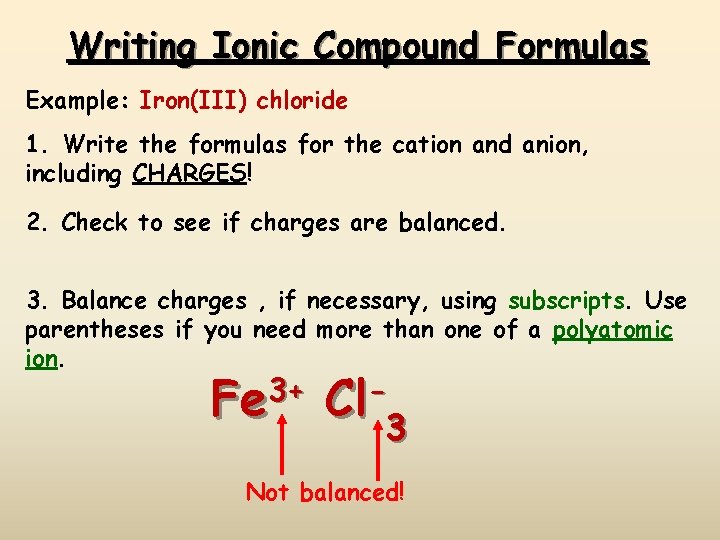
Writing Ionic Compound Formulas Example: Iron(III) chloride 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Fe 3+ Cl 3 3 Not balanced!

Writing Ionic Compound Formulas Example: Aluminum sulfide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. 3+ Al 2 2 S 3 Not balanced!

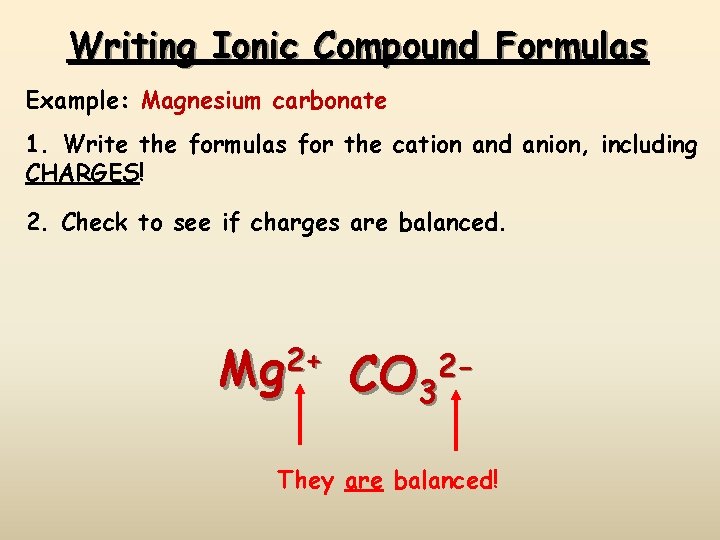
Writing Ionic Compound Formulas Example: Magnesium carbonate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 2+ Mg CO 32 - They are balanced!

Writing Ionic Compound Formulas Example: Zinc hydroxide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Zn 2+ ( OH- ) 2 Not balanced!

Writing Ionic Compound Formulas Example: Aluminum phosphate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3+ Al 3 PO 4 They ARE balanced!

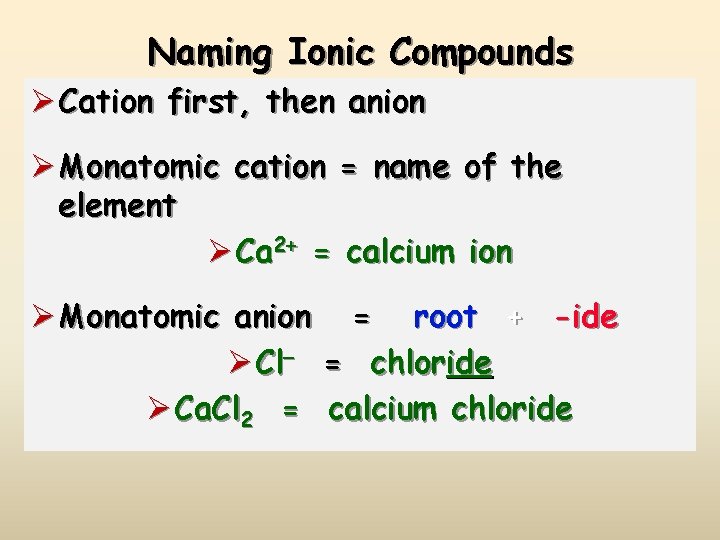
Naming Ionic Compounds Ø Cation first, then anion Ø Monatomic cation = name of the element Ø Ca 2+ = calcium ion Ø Monatomic anion = root + -ide Ø Cl- = chloride Ø Ca. Cl 2 = calcium chloride

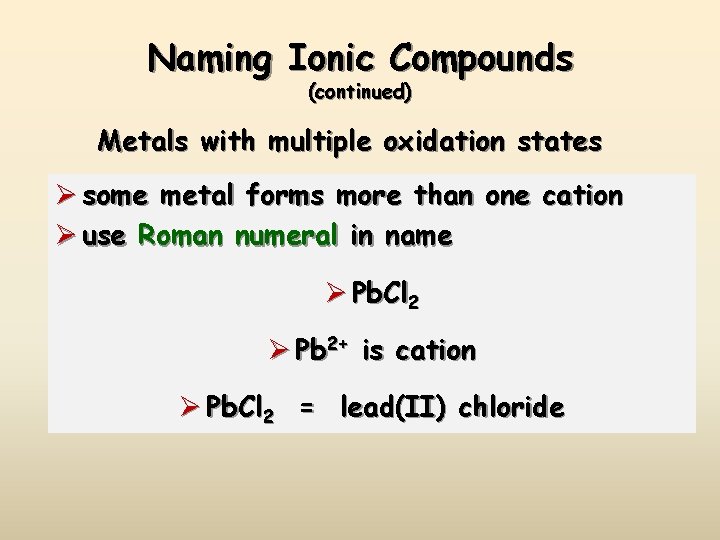
Naming Ionic Compounds (continued) Metals with multiple oxidation states Ø some metal forms more than one cation Ø use Roman numeral in name Ø Pb. Cl 2 Ø Pb 2+ is cation Ø Pb. Cl 2 = lead(II) chloride

Try these • Write names for the following compounds. Ca. CO 3 Al 2(SO 4)3 Ca(Cl. O 3)2 K 3 PO 4 ü Mg(OH)2 ü ü

Now, these! • Write formulas for the following compounds. ü ü ü Lithium sulfate Calcium acetate Aluminum nitrate Magnesium phosphate Sodium carbonate