Diagrammatic Representation of Atoms Year 11 Chemistry Last











- Slides: 42

Diagrammatic Representation of Atoms Year 11 Chemistry

Last Lesson • Isotopes • Calculating protons, neutrons and electrons • Mixtures & Pure substances • Homogeneous and heterogeneous mixtures • Electron arrangement • Valence electrons • Valence shells

Glossary • • Metals Non-metals Metalloids Isotopes Nomenclature Pure substance Mixture • Heterogeneous mixture • Homogeneous mixture • Electron configuration • Valence electrons • Bohr Diagrams • Lewis dot diagrams • Electronegativity

This lesson • Lewis Dot Diagrams • You will learn to draw LDD to represent atoms and compounds • Electronegativity • Learn how electronegativity effects bonding behaviour of elements

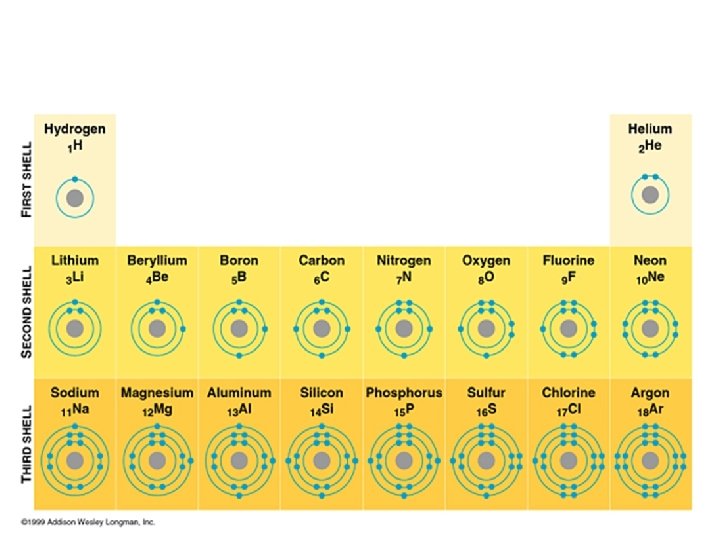
Bohr Diagrams • Represent the atom and its electrons in their entirety • All electrons in all shells are included

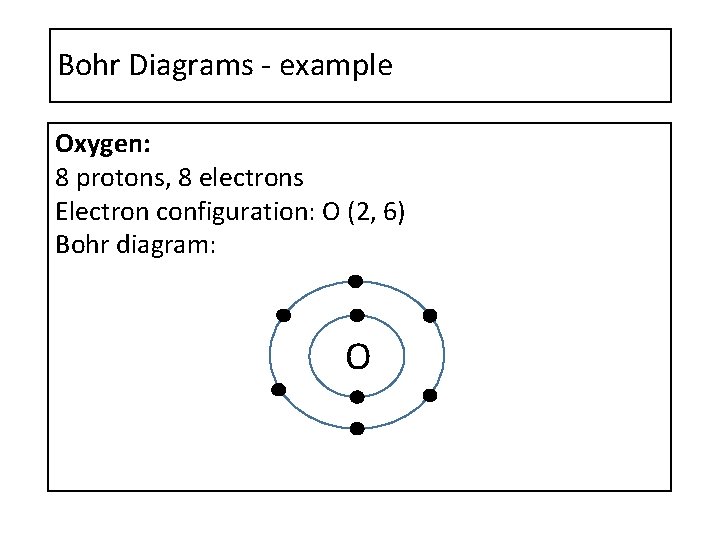
Bohr Diagrams - example Oxygen: 8 protons, 8 electrons Electron configuration: O (2, 6) Bohr diagram: O


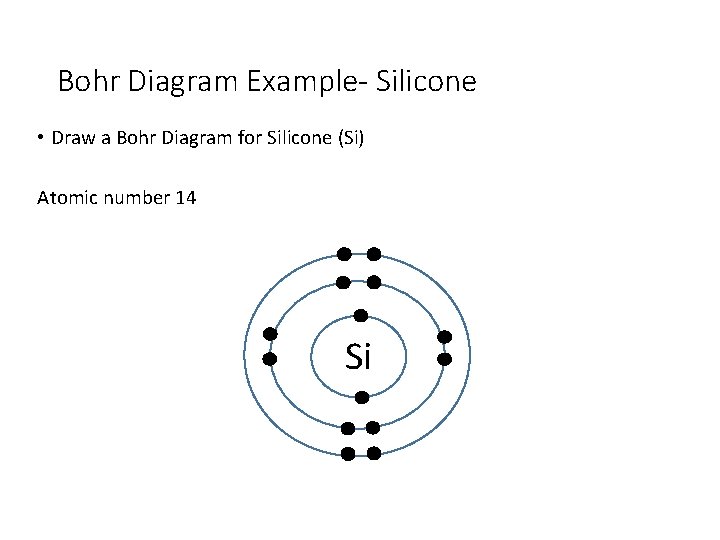
Bohr Diagram Example- Silicone • Draw a Bohr Diagram for Silicone (Si) Atomic number 14 Si

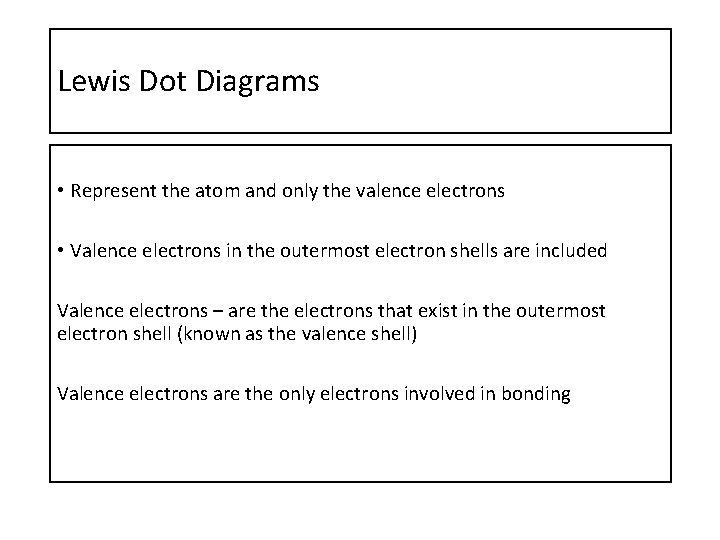
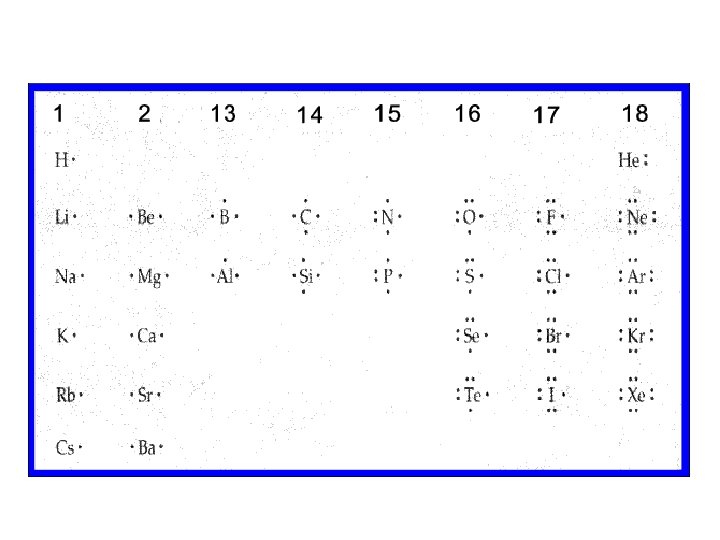
Lewis Dot Diagrams • Represent the atom and only the valence electrons • Valence electrons in the outermost electron shells are included Valence electrons – are the electrons that exist in the outermost electron shell (known as the valence shell) Valence electrons are the only electrons involved in bonding

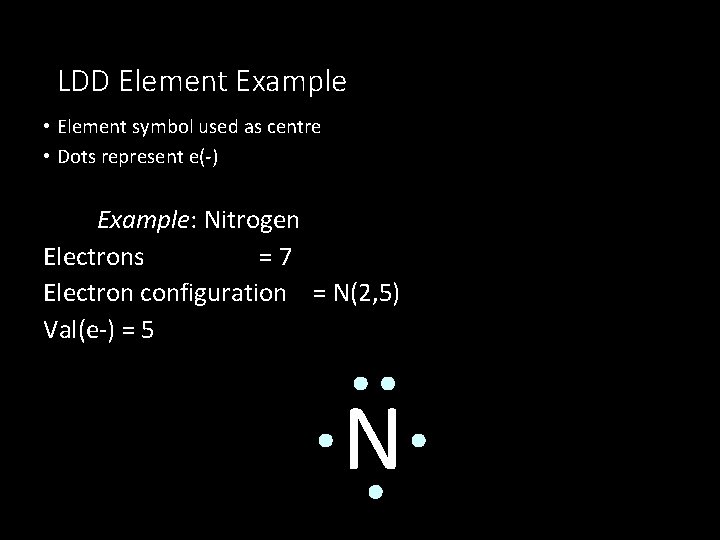
LDD Element Example • Element symbol used as centre • Dots represent e(-) Example: Nitrogen Electrons =7 Electron configuration = N(2, 5) Val(e-) = 5 N

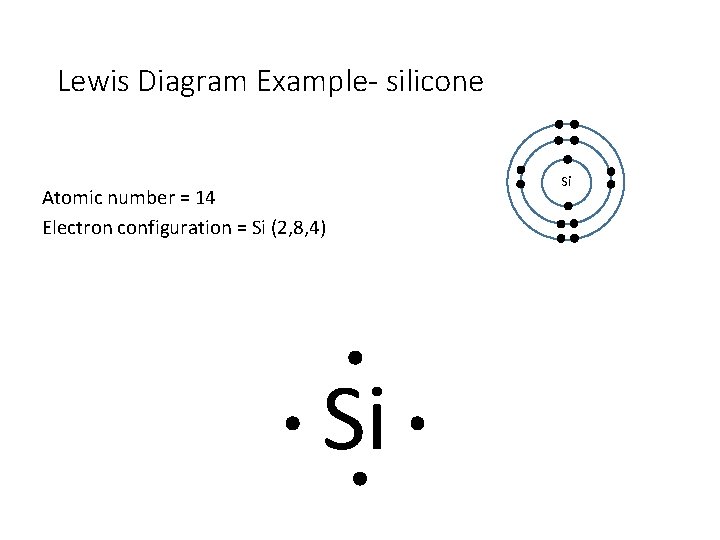
Lewis Diagram Example- silicone Atomic number = 14 Electron configuration = Si (2, 8, 4) Si Si


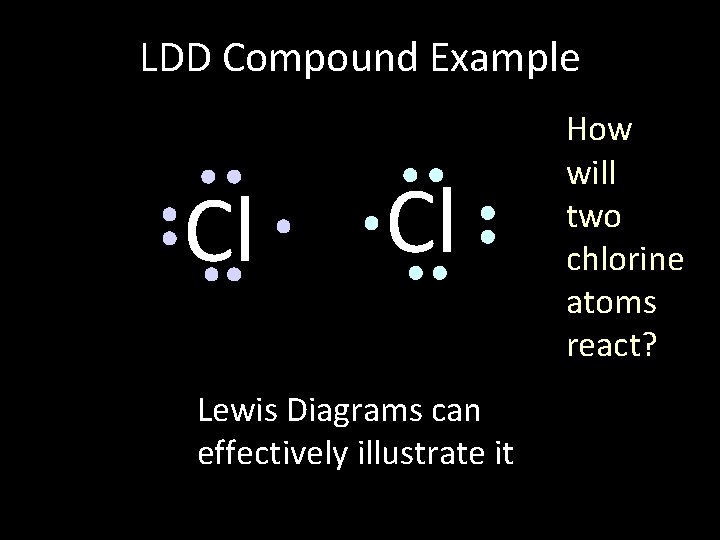
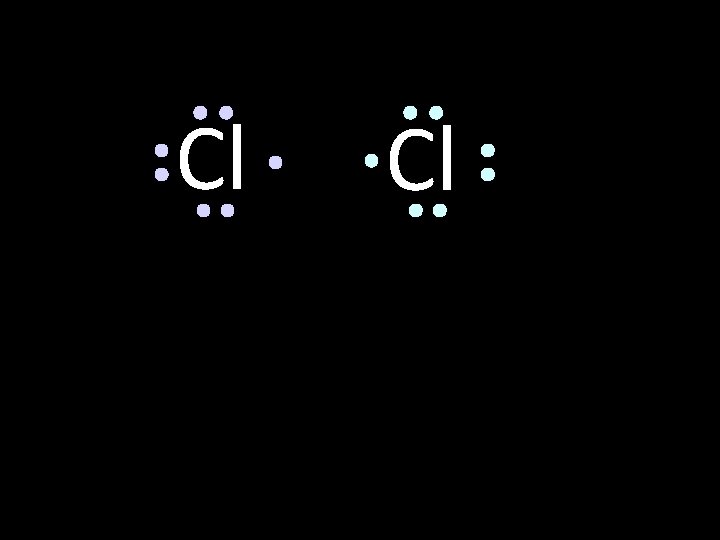
LDD Compound Example Cl Cl Lewis Diagrams can effectively illustrate it How will two chlorine atoms react?

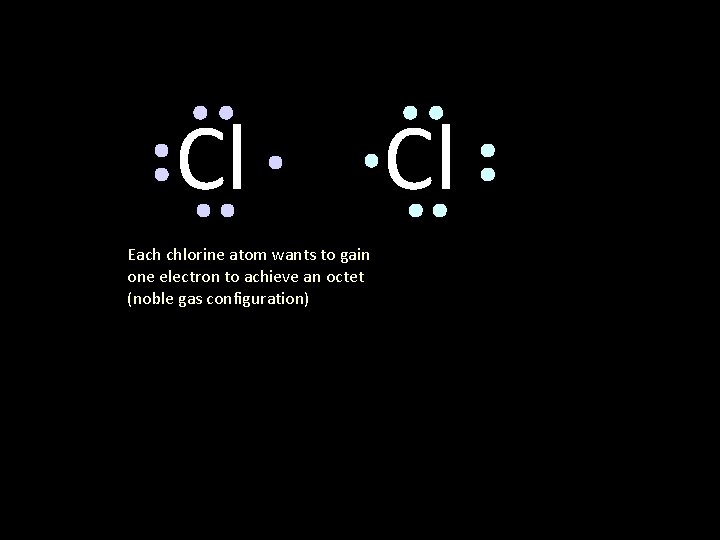
Cl Each chlorine atom wants to gain one electron to achieve an octet (noble gas configuration) Cl

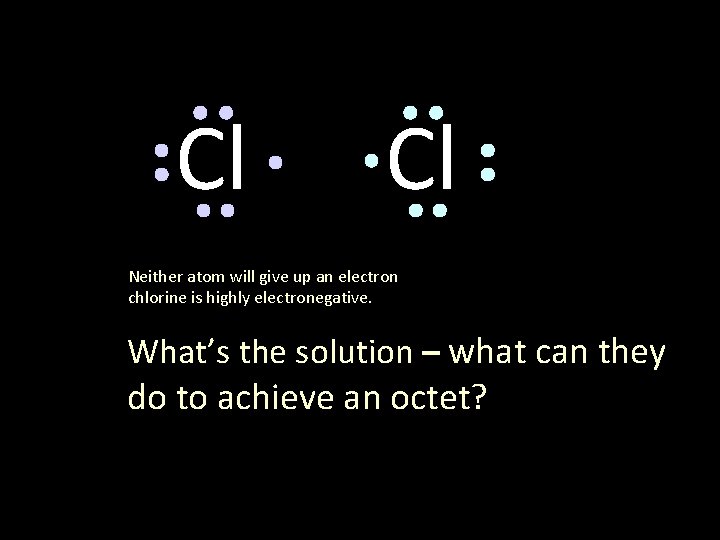
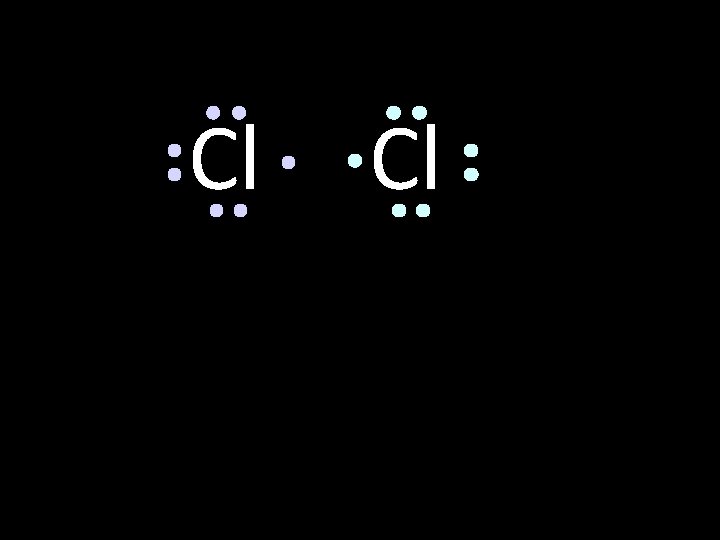
Cl Cl Neither atom will give up an electron chlorine is highly electronegative. What’s the solution – what can they do to achieve an octet?

Cl Cl

Cl Cl

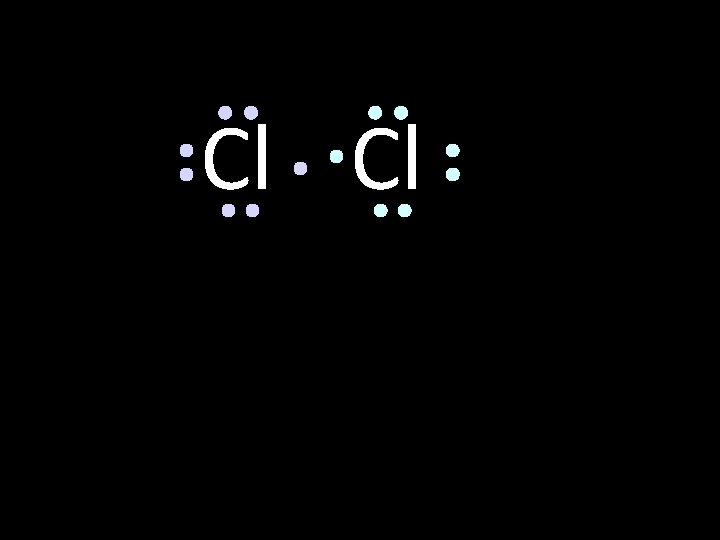
Cl Cl

Cl Cl

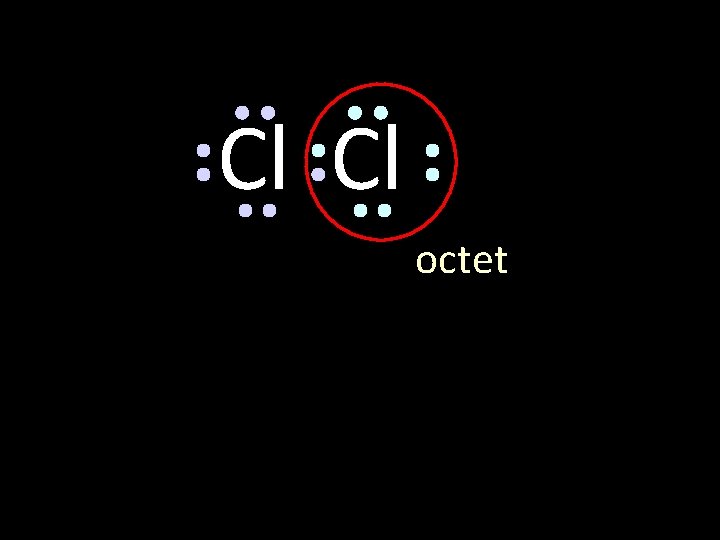
Cl Cl octet

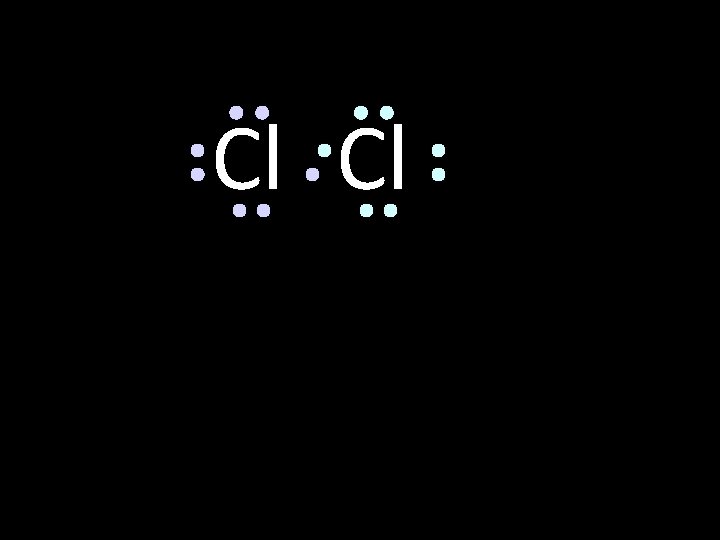
Cl Cl octet circle the electrons for each atom that completes their octets

Cl Cl The octet is achieved by each atom sharing the electron pair in the middle circle the electrons for each atom that completes their octets

Cl Cl The octet is achieved by each atom sharing the electron pair in the middle circle the electrons for each atom that completes their octets

Cl Cl This is the bonding pair circle the electrons for each atom that completes their octets

Activity • Bohr and Lewis

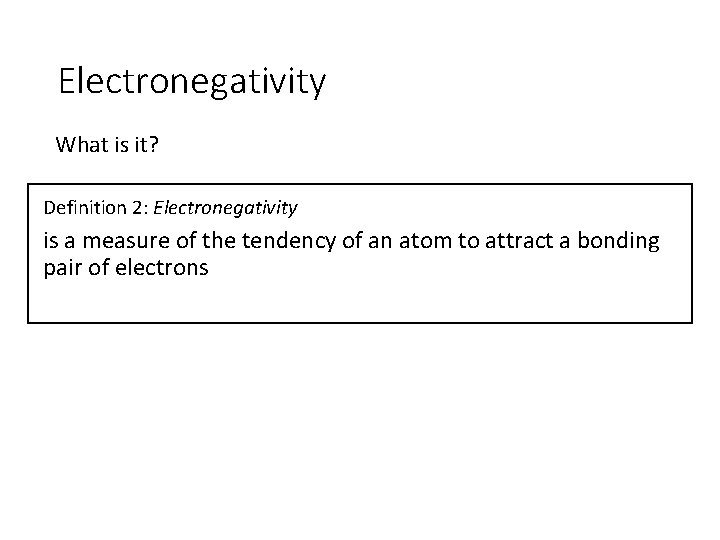
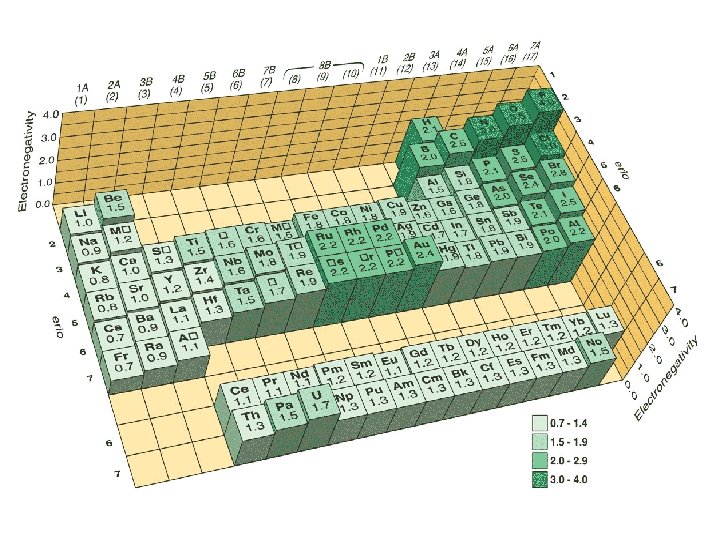
Electronegativity What is it? Definition 2: Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons

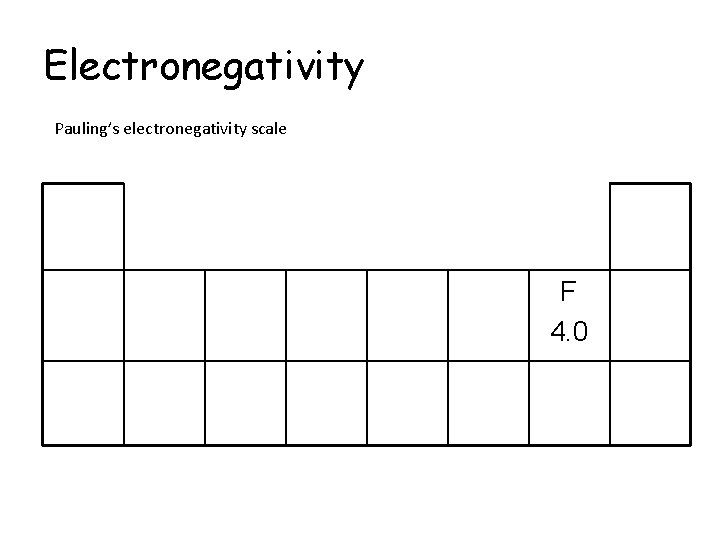
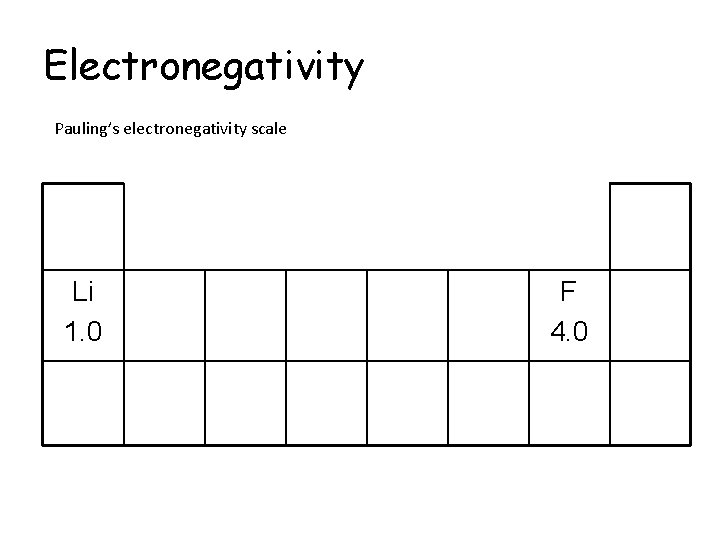
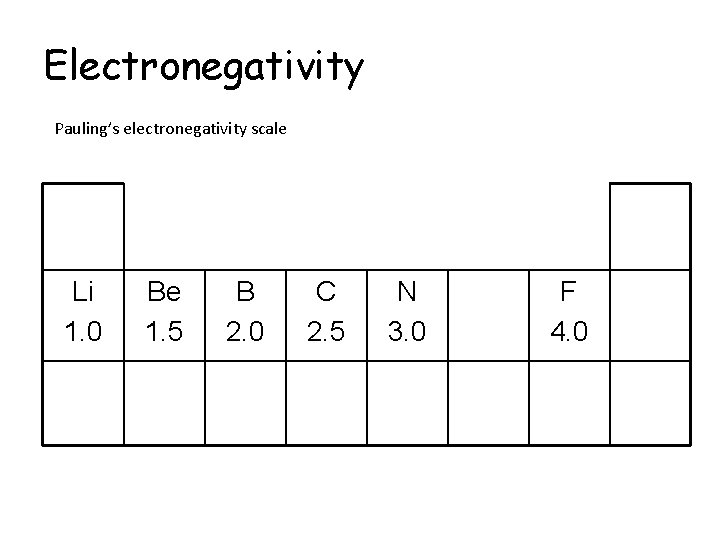
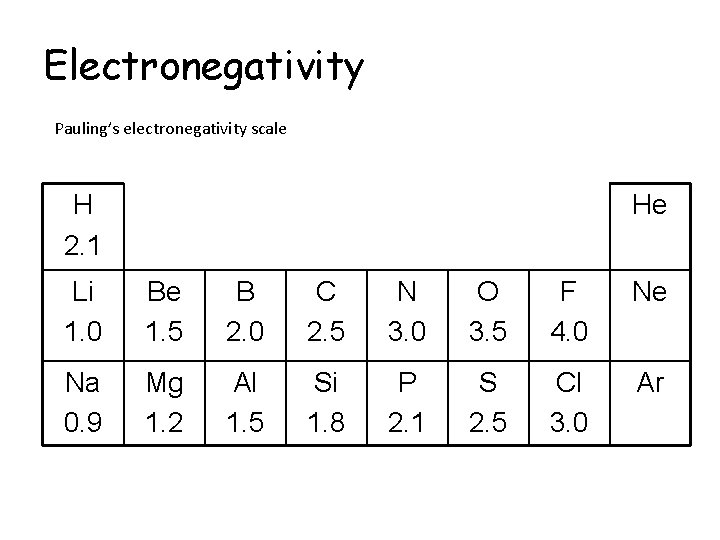
Electronegativity Pauling’s electronegativity scale The higher the value, the more electronegative the element Fluorine is the most electronegative element with an electronegativity value of 4. 0

Electronegativity Pauling’s electronegativity scale F

Electronegativity Pauling’s electronegativity scale F 4. 0

Electronegativity Pauling’s electronegativity scale Li 1. 0 F 4. 0

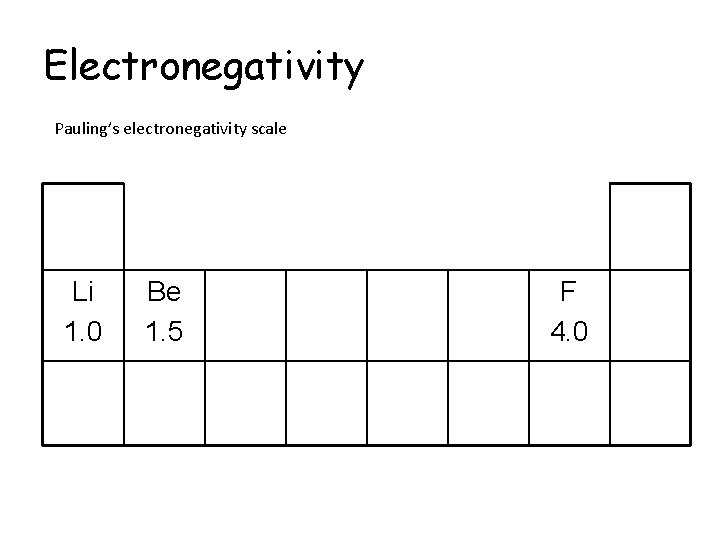
Electronegativity Pauling’s electronegativity scale Li 1. 0 Be 1. 5 F 4. 0

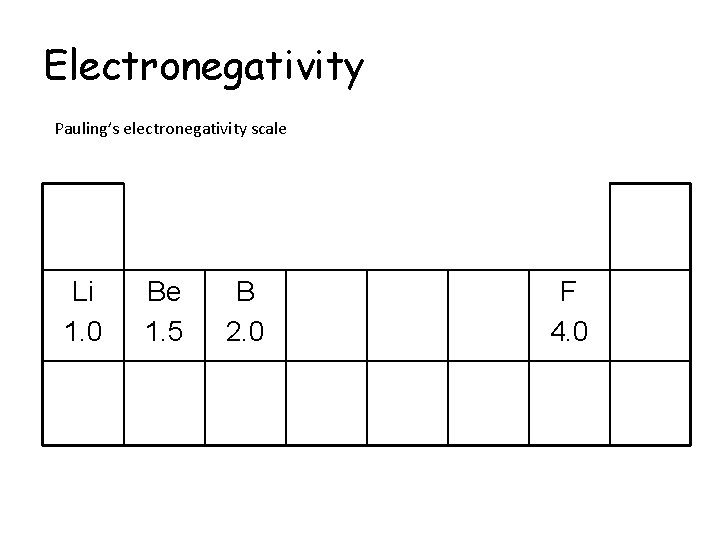
Electronegativity Pauling’s electronegativity scale Li 1. 0 Be 1. 5 B 2. 0 F 4. 0

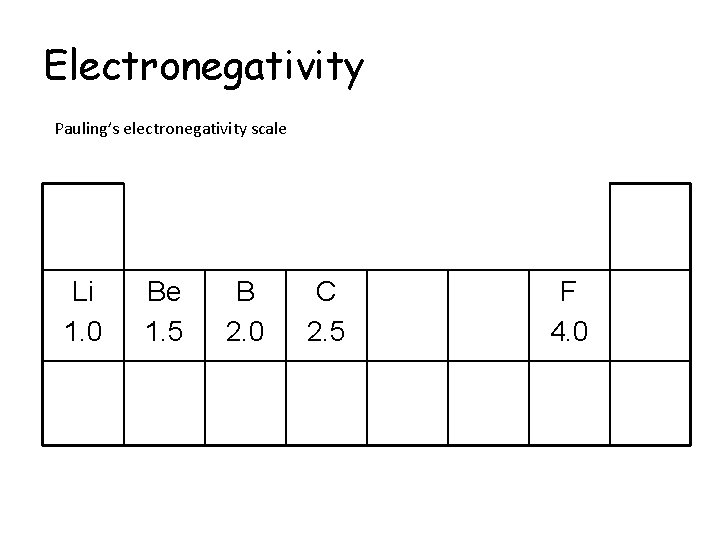
Electronegativity Pauling’s electronegativity scale Li 1. 0 Be 1. 5 B 2. 0 C 2. 5 F 4. 0

Electronegativity Pauling’s electronegativity scale Li 1. 0 Be 1. 5 B 2. 0 C 2. 5 N 3. 0 F 4. 0

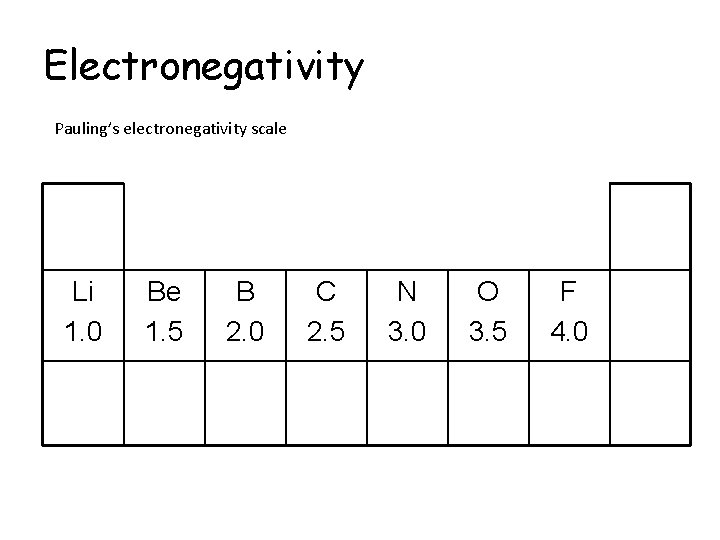
Electronegativity Pauling’s electronegativity scale Li 1. 0 Be 1. 5 B 2. 0 C 2. 5 N 3. 0 O 3. 5 F 4. 0

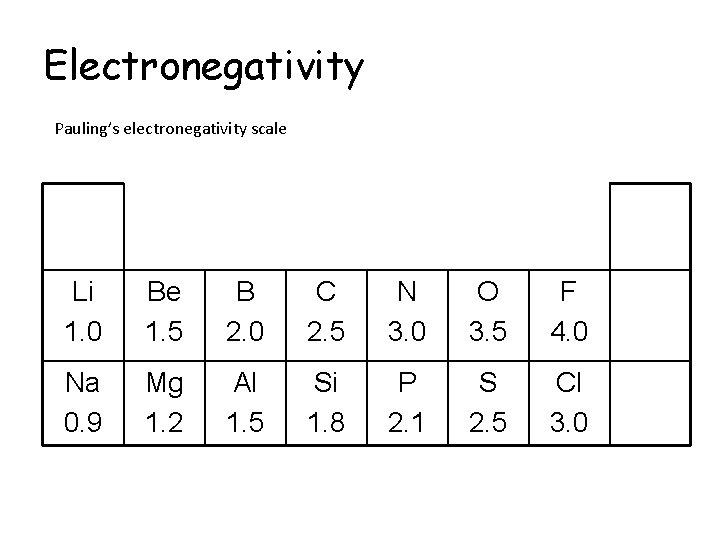
Electronegativity Pauling’s electronegativity scale Li 1. 0 Be 1. 5 B 2. 0 C 2. 5 N 3. 0 O 3. 5 F 4. 0 Na 0. 9 Mg 1. 2 Al 1. 5 Si 1. 8 P 2. 1 S 2. 5 Cl 3. 0

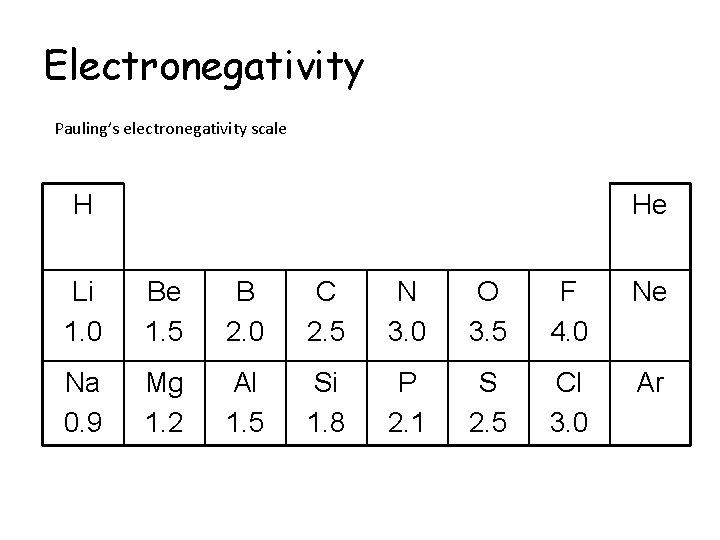
Electronegativity Pauling’s electronegativity scale H He Li 1. 0 Be 1. 5 B 2. 0 C 2. 5 N 3. 0 O 3. 5 F 4. 0 Ne Na 0. 9 Mg 1. 2 Al 1. 5 Si 1. 8 P 2. 1 S 2. 5 Cl 3. 0 Ar

Electronegativity Pauling’s electronegativity scale H 2. 1 He Li 1. 0 Be 1. 5 B 2. 0 C 2. 5 N 3. 0 O 3. 5 F 4. 0 Ne Na 0. 9 Mg 1. 2 Al 1. 5 Si 1. 8 P 2. 1 S 2. 5 Cl 3. 0 Ar

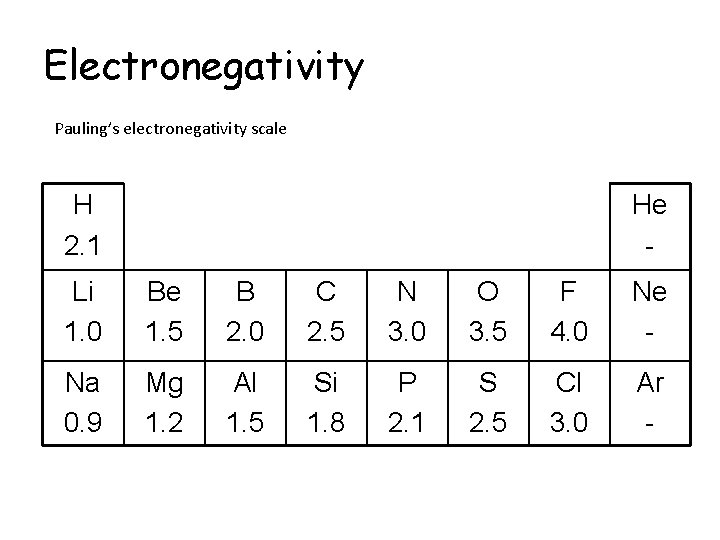
Electronegativity Pauling’s electronegativity scale H 2. 1 He - Li 1. 0 Be 1. 5 B 2. 0 C 2. 5 N 3. 0 O 3. 5 F 4. 0 Ne - Na 0. 9 Mg 1. 2 Al 1. 5 Si 1. 8 P 2. 1 S 2. 5 Cl 3. 0 Ar -


Glossary • • Metals Non-metals Metalloids Isotopes Nomenclature Pure substance Mixture • Heterogeneous mixture • Homogeneous mixture • Electron configuration • Valence electrons • Bohr Diagrams • Lewis dot diagrams • Electronegativity

Glossary so far. . . • Atom • Element • Molecule • Compound • Ion • Isotope • Periodic table • Sub-atomic particles • Protons (+) • Electrons (-) • Neutrons • Atomic number • Atomic mass • Metals • Non-metals • Metalloids • Isotopes • Pure substance • Mixtures • Heterogeneous • Homogeneous • Electron configuration • Valence electrons • Bohr diagrams • Lewis diagrams • Electronegativity