diagonal rule The Diagonal Rule A simple use


- Slides: 14

diagonal rule The Diagonal Rule A simple use of 2 quantum numbers 1

diagonal rule Summary of Bohr’s Model (1913) Electrons are in different orbits at fixed distances from nucleus. Electrons that leave one orbit must move to another orbit. Electrons only change orbits if specific amounts (quanta) of extra energy from the outside world are involved. Electrons that receive enough extra energy from the outside world can leave the atom they are in. Electrons that return to orbits they used to reside in give up the extra energy they acquired when they moved in the first place. Electronic energy given up when electrons move back into an original orbit often shows up as a specific color light. 2

diagonal rule Light is emitted from an atom when electrons in an atom move from an excited state configuration to the atom’s ground state configuration. The lowest orbital (energy) arrangement for the electrons around the nucleus of an atom is know as the ground state of the atom. Electron arrangements about the nucleus that are not the lowest possible energy arrangement are know as excited states. The diagonal rule helps technicians, engineers and scientists determine the ground state arrangement of electrons about the nucleus of an atom. 3

diagonal rule Light is emitted from an atom when electrons in an atom move from an excited state configuration to the atom’s ground state configuration. The diagonal rule uses the first two quantum numbers The diagonal rule helps technicians, engineers and scientists determine the ground state arrangement of electrons about the nucleus of an atom. 4

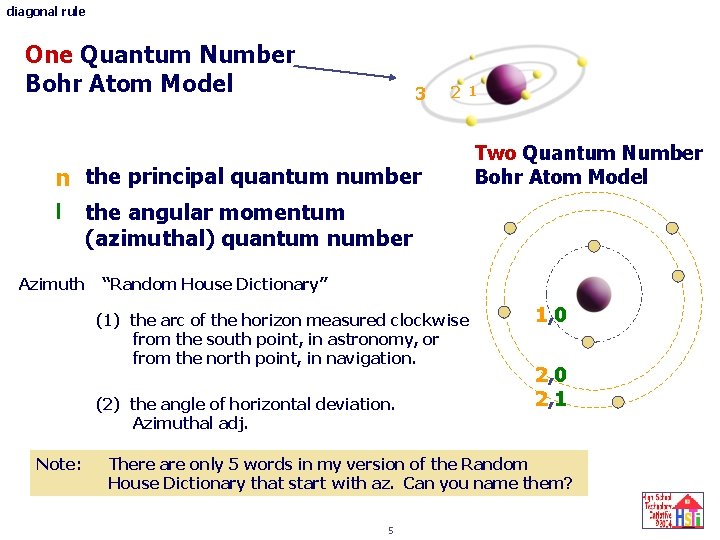
diagonal rule One Quantum Number Bohr Atom Model 3 2 1 n the principal quantum number l the angular momentum Two Quantum Number Bohr Atom Model (azimuthal) quantum number Azimuth “Random House Dictionary” (1) the arc of the horizon measured clockwise from the south point, in astronomy, or from the north point, in navigation. (2) the angle of horizontal deviation. Azimuthal adj. Note: 1, 0 2, 1 There are only 5 words in my version of the Random House Dictionary that start with az. Can you name them? 5

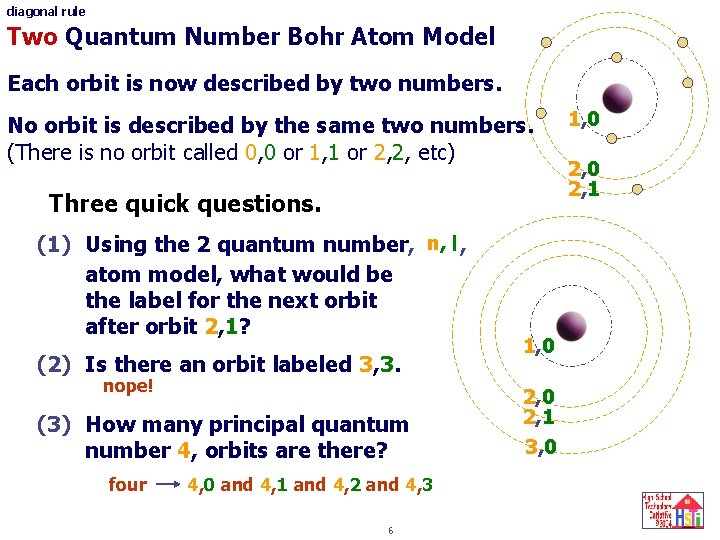
diagonal rule Two Quantum Number Bohr Atom Model Each orbit is now described by two numbers. No orbit is described by the same two numbers. (There is no orbit called 0, 0 or 1, 1 or 2, 2, etc) Three quick questions. (1) Using the 2 quantum number, n, l , atom model, what would be the label for the next orbit after orbit 2, 1? (2) Is there an orbit labeled 3, 3. nope! (3) How many principal quantum number 4, orbits are there? four 4, 0 and 4, 1 and 4, 2 and 4, 3 6 1, 0 2, 1 3, 0 1, 0 2, 1

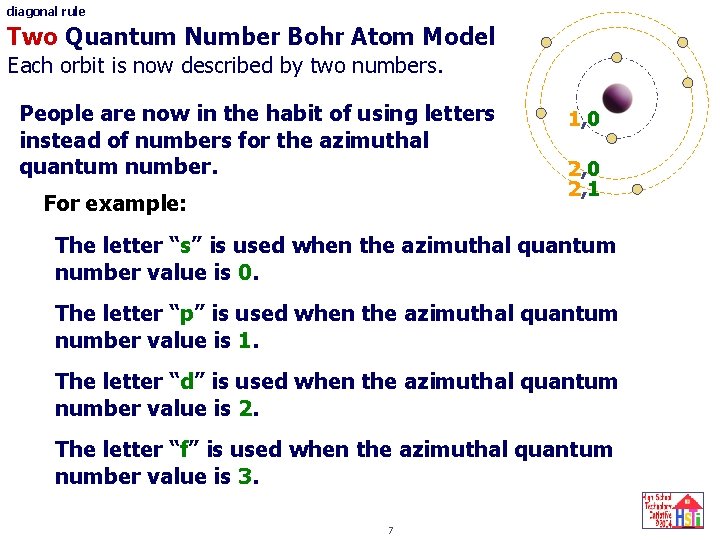
diagonal rule Two Quantum Number Bohr Atom Model Each orbit is now described by two numbers. People are now in the habit of using letters instead of numbers for the azimuthal quantum number. For example: 1, 0 2, 1 The letter “s” is used when the azimuthal quantum number value is 0. The letter “p” is used when the azimuthal quantum number value is 1. The letter “d” is used when the azimuthal quantum number value is 2. The letter “f” is used when the azimuthal quantum number value is 3. 7

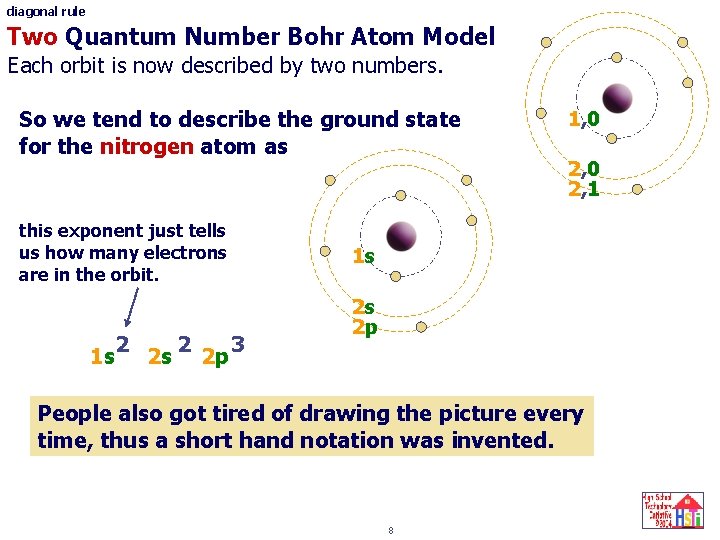
diagonal rule Two Quantum Number Bohr Atom Model Each orbit is now described by two numbers. So we tend to describe the ground state for the nitrogen atom as this exponent just tells us how many electrons are in the orbit. 2 2 3 1 s 2 s 2 p 1, 0 2, 1 1 s 2 s 2 p People also got tired of drawing the picture every time, thus a short hand notation was invented. 8

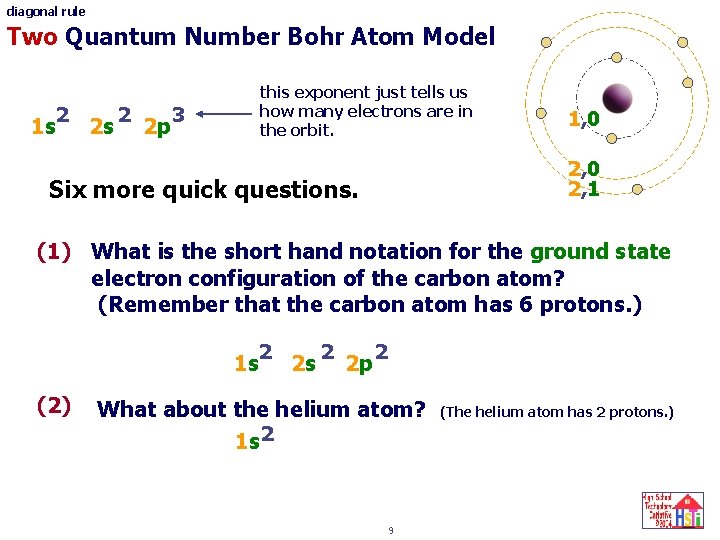
diagonal rule Two Quantum Number Bohr Atom Model 2 1 s 2 s 2 2 p this exponent just tells us how many electrons are in the orbit. 3 1, 0 2, 1 Six more quick questions. (1) What is the short hand notation for the ground state electron configuration of the carbon atom? (Remember that the carbon atom has 6 protons. ) 2 1 s (2) 2 s 2 2 p 2 What about the helium atom? 1 s 2 9 (The helium atom has 2 protons. )

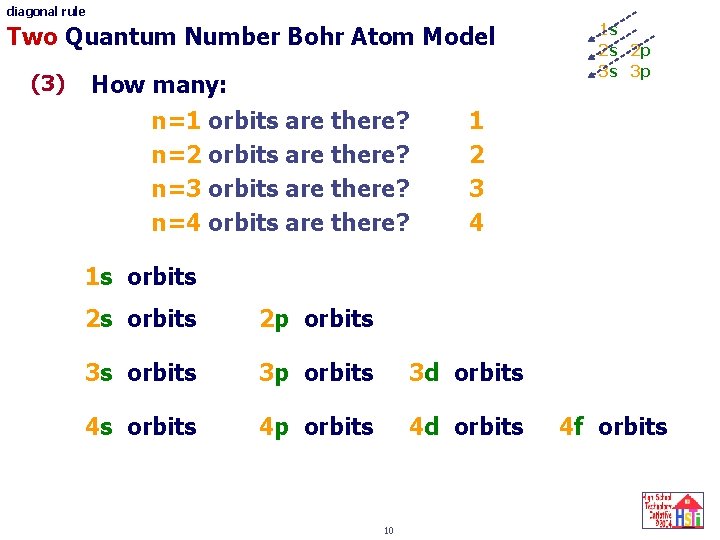
diagonal rule Two Quantum Number Bohr Atom Model (3) How many: n=1 orbits are there? n=2 orbits are there? n=3 orbits are there? n=4 orbits are there? 1 s 2 s 2 p 3 s 3 p 1 2 3 4 1 s orbits 2 p orbits 3 s orbits 3 p orbits 3 d orbits 4 s orbits 4 p orbits 4 d orbits 10 4 f orbits

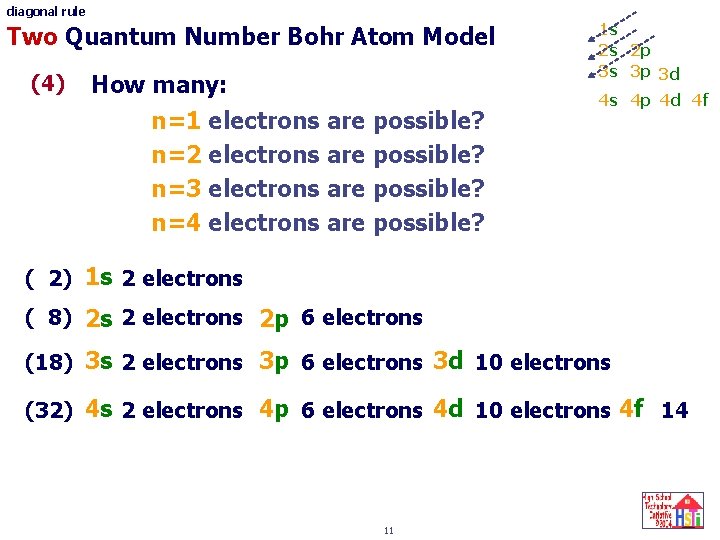
diagonal rule Two Quantum Number Bohr Atom Model (4) How many: n=1 electrons are possible? n=2 electrons are possible? n=3 electrons are possible? n=4 electrons are possible? 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f ( 2) 1 s 2 electrons ( 8) 2 s 2 electrons 2 p 6 electrons (18) 3 s 2 electrons 3 p 6 electrons 3 d 10 electrons (32) 4 s 2 electrons 4 p 6 electrons 4 d 10 electrons 4 f 14 ? 11

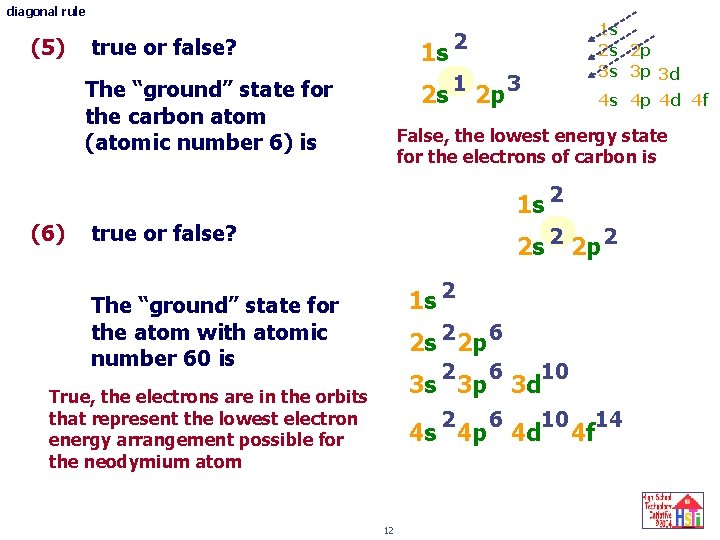
diagonal rule (5) true or false? 1 s 2 The “ground” state for the carbon atom (atomic number 6) is 2 s 1 2 p 3 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f False, the lowest energy state for the electrons of carbon is 1 s 2 (6) true or false? 2 s 2 2 p 2 1 s 2 The “ground” state for the atom with atomic number 60 is 2 s 2 2 p 6 2 6 10 3 s 3 p 3 d True, the electrons are in the orbits that represent the lowest electron energy arrangement possible for the neodymium atom 4 s 4 p 4 d 12 14 4 f

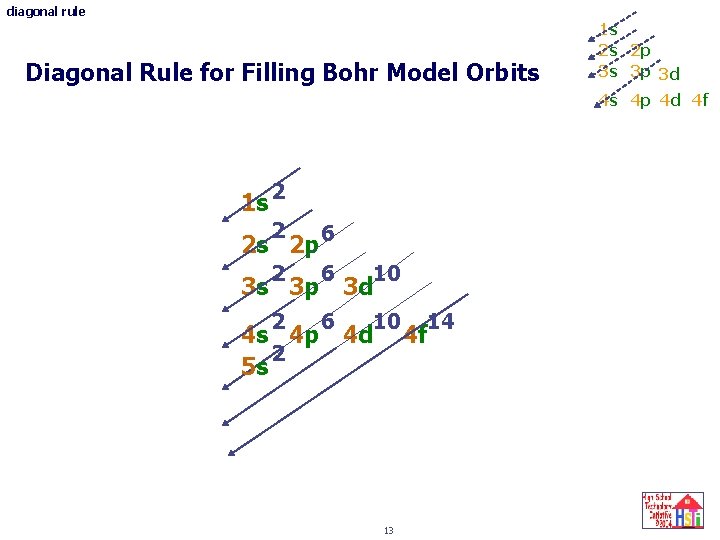
diagonal rule Diagonal Rule for Filling Bohr Model Orbits 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 1 s 2 2 2 s 2 p 6 2 6 10 3 s 3 p 3 d 4 s 4 p 4 d 2 5 s 13 14 4 f

diagonal rule 14