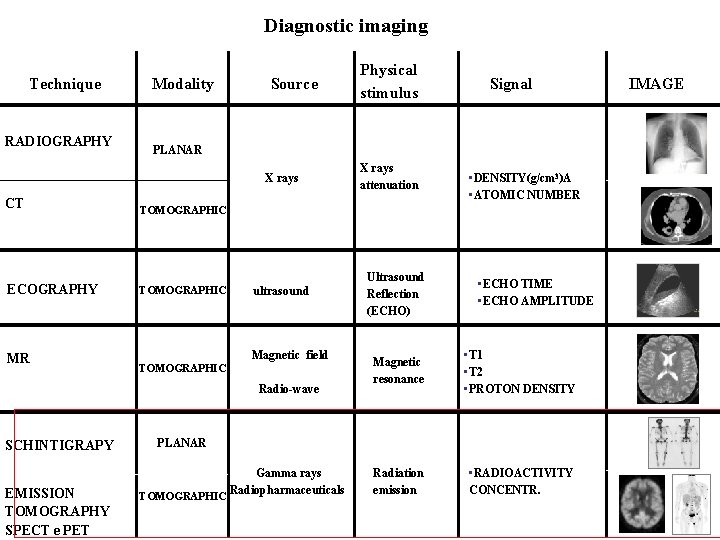
Diagnostic imaging Technique RADIOGRAPHY Modality Source CT TOMOGRAPHIC
![NUCLEAR MEDICINE DIAGNOSTIC IMAGING [99 m. Tc]MDP scintigrafia planare [18 F]FDG - PET Th. NUCLEAR MEDICINE DIAGNOSTIC IMAGING [99 m. Tc]MDP scintigrafia planare [18 F]FDG - PET Th.](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-2.jpg)











![[18 F]fluorodeoxyglucose BBB tissue plasma [F-18]FDG Cp F-18]FDG-P No to * Fructose -6 -P [18 F]fluorodeoxyglucose BBB tissue plasma [F-18]FDG Cp F-18]FDG-P No to * Fructose -6 -P](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-45.jpg)
![Binding • Recettor or transporters: • • [11 C]Raclopride- DA-D 2 like rec [123] Binding • Recettor or transporters: • • [11 C]Raclopride- DA-D 2 like rec [123]](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-47.jpg)

![3’-[18 F]fluoro-3’-deossitimidina ([18 F]FLT) Cell proliferation marker. Tymidine kinase I add a P group 3’-[18 F]fluoro-3’-deossitimidina ([18 F]FLT) Cell proliferation marker. Tymidine kinase I add a P group](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-52.jpg)

![[18 F]FDG: GLUCOSE ANALOGUE n. Transported as glucose with GLUx transporter n. Phosphorilated in [18 F]FDG: GLUCOSE ANALOGUE n. Transported as glucose with GLUx transporter n. Phosphorilated in](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-61.jpg)
![[18 F]FDG as an example Deoxyglucose is STOPED AS 6 PHOSPHATE EXOSES [18 F]FDG as an example Deoxyglucose is STOPED AS 6 PHOSPHATE EXOSES](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-62.jpg)
![2 -[F-18]fluoro-2 -deoxy-D-Glucose [F-18]FDG) GLUT FDG blood Brain retention of FDG is dependent on 2 -[F-18]fluoro-2 -deoxy-D-Glucose [F-18]FDG) GLUT FDG blood Brain retention of FDG is dependent on](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-63.jpg)
![[18 F]FDG humans Fasting conditions [18 F]FDG humans Fasting conditions](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-65.jpg)
![Why [18 F]FDGi n cancer? Why [18 F]FDGi n cancer?](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-69.jpg)
![Why [18 F]FDGi n cancer? n Higher glycolisis (Warburg effect: increase in glycolis in Why [18 F]FDGi n cancer? n Higher glycolisis (Warburg effect: increase in glycolis in](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-70.jpg)
- Slides: 73

Diagnostic imaging Technique RADIOGRAPHY Modality Source CT TOMOGRAPHIC ECOGRAPHY TOMOGRAPHIC ultrasound Magnetic field TOMOGRAPHIC Radio-wave SCHINTIGRAPY EMISSION TOMOGRAPHY SPECT e PET Signal PLANAR X rays MR Physical stimulus X rays attenuation Ultrasound Reflection (ECHO) • DENSITY(g/cm 3)A • ATOMIC NUMBER • ECHO TIME • ECHO AMPLITUDE Magnetic resonance • T 1 • T 2 • PROTON DENSITY Radiation emission • RADIOACTIVITY CONCENTR. PLANAR Gamma rays Radiopharmaceuticals TOMOGRAPHIC IMAGE
![NUCLEAR MEDICINE DIAGNOSTIC IMAGING 99 m TcMDP scintigrafia planare 18 FFDG PET Th NUCLEAR MEDICINE DIAGNOSTIC IMAGING [99 m. Tc]MDP scintigrafia planare [18 F]FDG - PET Th.](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-2.jpg)
NUCLEAR MEDICINE DIAGNOSTIC IMAGING [99 m. Tc]MDP scintigrafia planare [18 F]FDG - PET Th. ERAPY [131 I]Na. I: scintigrafia/terapia No images without a Radiopharmaceutical except for radiation internal contaminations

I radiopharmaceutical on the basis of the EU Directive D. L. vo n. 178 del 29/5/91, are classified as medicinal products. Italian version: ……. . è da intendersi come medicinale ogni sostanza o composizione presentata come avente proprietà curative o profilattiche delle malattie umane o animali, nonché ogni sostanza o composizione da somministrare all'uomo o all'animale allo scopo di stabilire una diagnosi medica o di ripristinare, correggere o modificare funzioni organiche dell'uomo o dell'animale.

RADIOPHARMACEUTICAS

. Aim: to carry radioactivity at target site to treat a clinical conditions (tumour) or to perform a diagnosis. A radiopharmaceutical need different kinetics and pharmacological properties depending on the use (therapy, diagnosis) or target localization (intracerebral, intracellular etc. . ). After administration (in general by i. v. injection) the molecule reachs the target organ but also others body districts that could interefere with the quality of the images obtained or in case of therapeutic use increase its toxicity Administration: intra venous

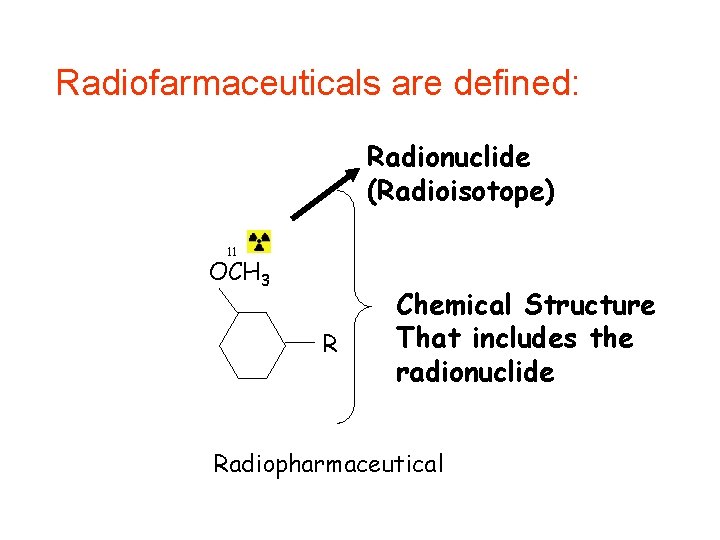
Radiofarmaceuticals are defined: Radionuclide (Radioisotope) 11 OCH 3 R Chemical Structure That includes the radionuclide Radiopharmaceutical

• Chemical structure: Kinetics of distribution, biological half life, target interaction.

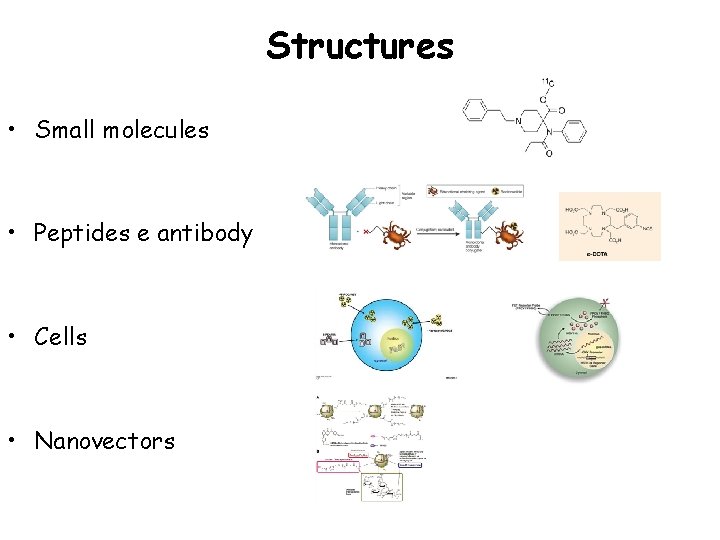
Structures • Small molecules • Peptides e antibody • Cells • Nanovectors Figure 1. Surface chelation model of 64 Cu-labeled

Radionuclides: applications Radiation emitted (type and energy), interaction with cells and tissues (radiotoxicity), half lìfe (time necessary to the 50% of atoms present in the sample decay and are transformed in an other element). • Diagnostic : high penetration and low interaction with tissues, energy adequate to be detected by medical devices. Radionuclides determin the acquisition device used and the temporal frame of the potential detection of the radiopharmaceutical (half-life) • Radioisotopes gamma emitters (planar scintigraphy, or tomographic scintigraphy SPECT): 123 I (10 h), 99 m. Tc (6 h)… • Radioisotopes beta + or positron emitters : 11 C (20. 38 min. ), 18 F (108. 9 min. ), 64 Cu (12. 7 h. ), 13 N(9. 97 min. ) • Therapeutic: high interaction with tissue, low penetration. • beta-: 90 Y, 188 Re , 131 I, 166 Lu • Diagnostic and therapy: 64 Cu (beta+ and beta -), 166 Lu (gamma and beta ) may be used for therapy and diagnostic

RADIONUCLIDE

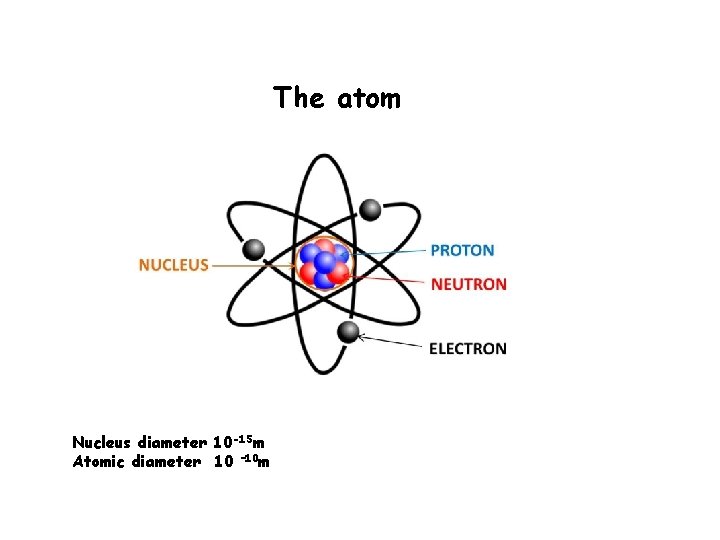
The atom Nucleus diameter 10 -15 m Atomic diameter 10 – 10 m

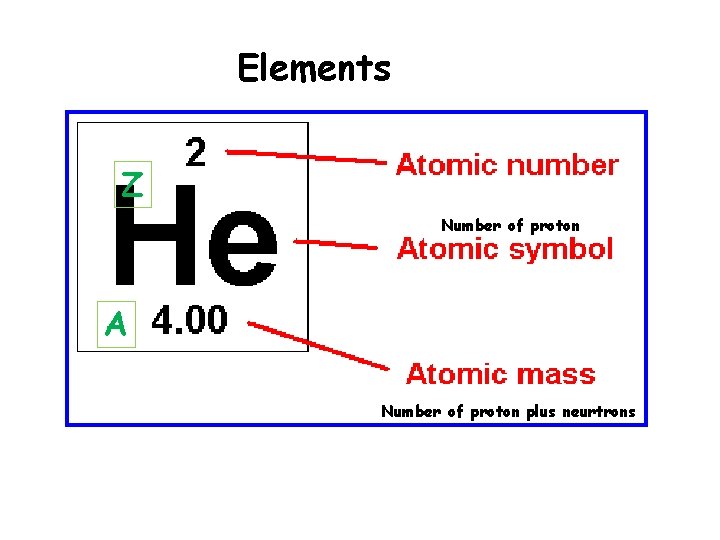
Elements Z Number of proton A Number of proton plus neurtrons

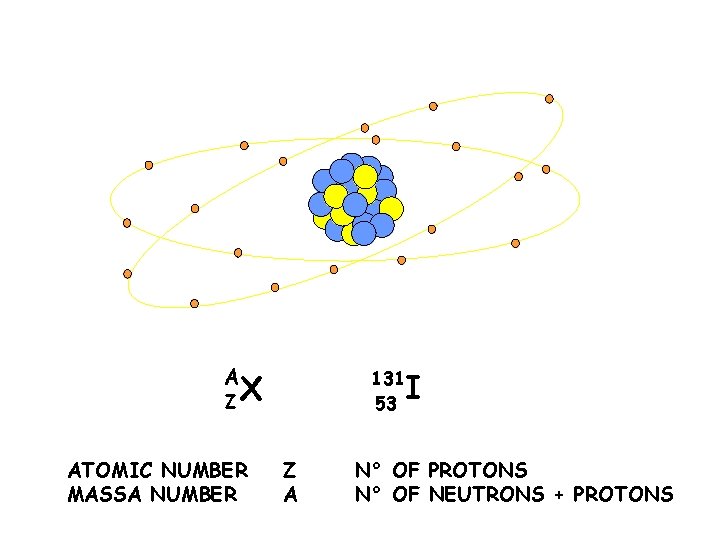
STRUTTURA DI UN NUCLEO A ZX ATOMIC NUMBER MASSA NUMBER 131 53 Z A I N° OF PROTONS N° OF NEUTRONS + PROTONS

Proton: positive charge particles, Kg: 1, 672 x 10 -27 Neutron, kg: 1, 675 x 10 -27 Electron: negative charge particles, kg: 9, 109 x 10 -31


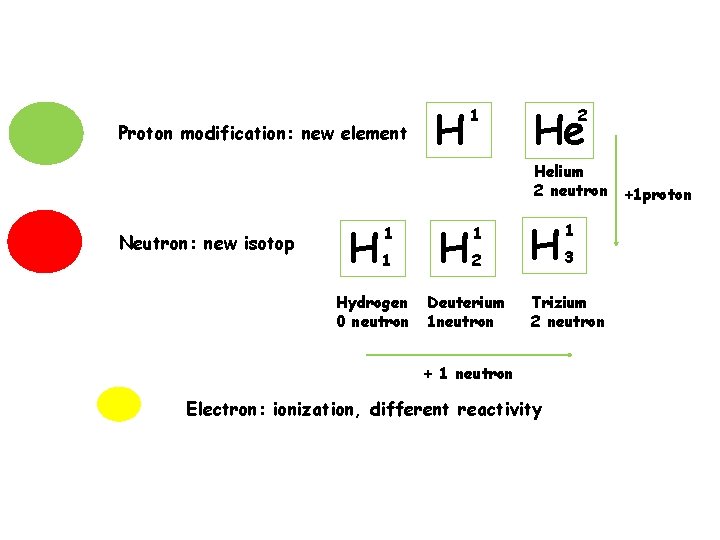
An element has the same number of proton An isotope of an element has the number of proton but a different number of neurtron

Proton modification: new element H 1 He 2 Helium 2 neutron +1 proton Neutron: new isotop H 1 1 Hydrogen 0 neutron H 1 2 Deuterium 1 neutron H 1 3 Trizium 2 neutron + 1 neutron Electron: ionization, different reactivity

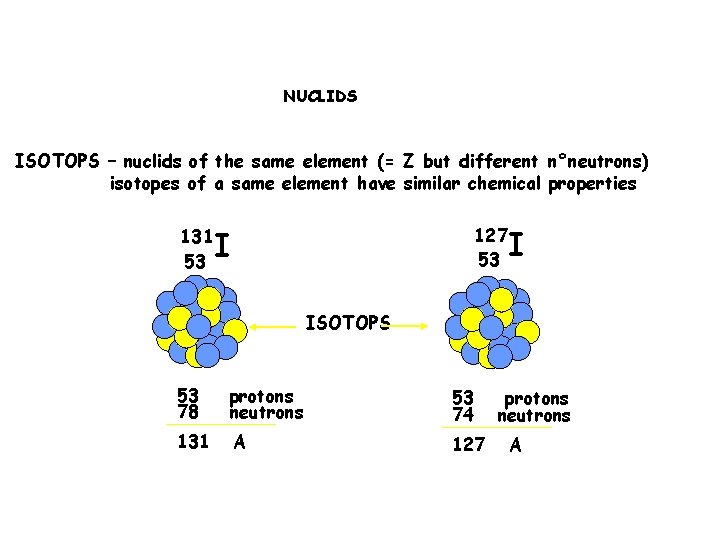
NUCLIDS ISOTOPS – nuclids of the same element (= Z but different n°neutrons) isotopes of a same element have similar chemical properties 131 53 127 53 I I ISOTOPS 53 78 protons neutrons 53 74 131 A 127 protons neutrons A

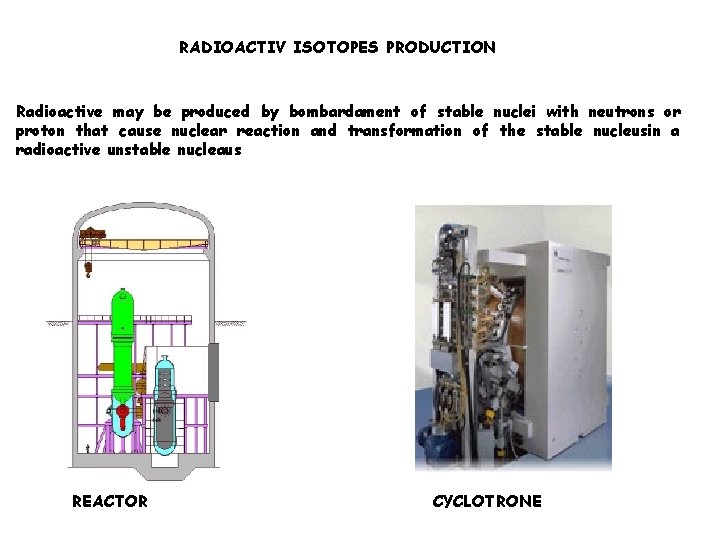
Radionuclides production: Reactor Cyclotron (internal use for radiolabeling or distribution) Generator (nuclear medicine radiopharmaceutical lab)

RADIOACTIV ISOTOPES PRODUCTION Radioactive may be produced by bombardament of stable nuclei with neutrons or proton that cause nuclear reaction and transformation of the stable nucleusin a radioactive unstable nucleaus REACTOR CYCLOTRONE

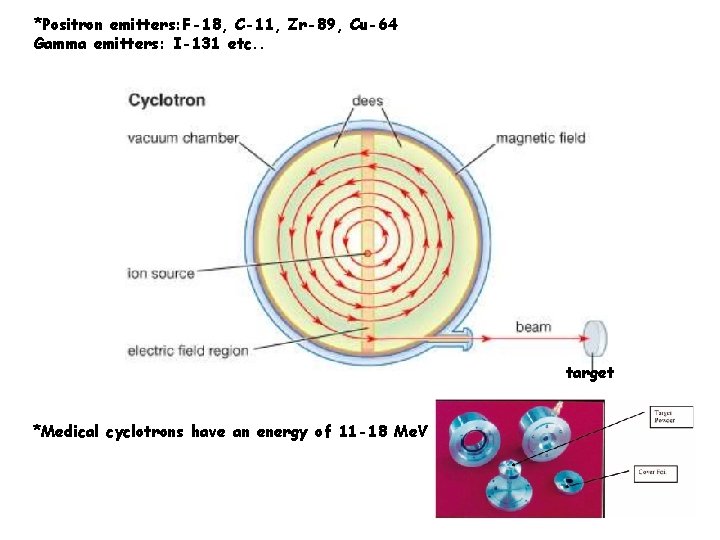
*Positron emitters: F-18, C-11, Zr-89, Cu-64 Gamma emitters: I-131 etc. . target *Medical cyclotrons have an energy of 11 -18 Me. V

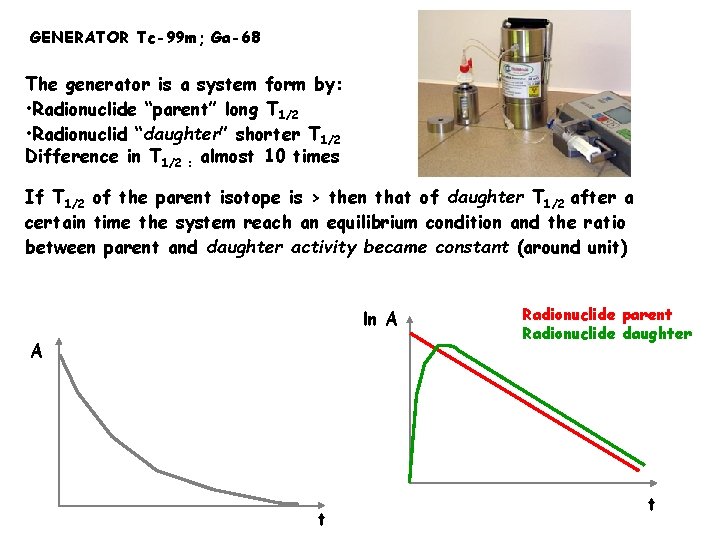
GENERATOR Tc-99 m; Ga-68 The generator is a system form by: • Radionuclide “parent” long T 1/2 • Radionuclid “daughter” shorter T 1/2 Difference in T 1/2 : almost 10 times If T 1/2 of the parent isotope is > then that of daughter T 1/2 after a certain time the system reach an equilibrium condition and the ratio between parent and daughter activity became constant (around unit) ln A A t Radionuclide parent Radionuclide daughter t

Kinetics and pharmacological properties

• Radiopharmaceuticals for diagnostic use: in vivo image or measure of a biological process that has a diagnostic meaning (or of research interest) – radiopharmaceutical = a tracer It traces a biologicical process without modify the system under study. Low mass administer and high sensitive methods requested (able to detect substances in concentration < n. M) – It is necessary a physical system able to detec radioation emitted • Radiopharmaceuticals for therapy: tissue damage specific for a certain type of district through the use of radiation emitter by an isotope carried by a molecule selective for a target preferentially expresed by the target tissue (cancer) and retained in that region for a time sufficient to exert its therapeutic activity.

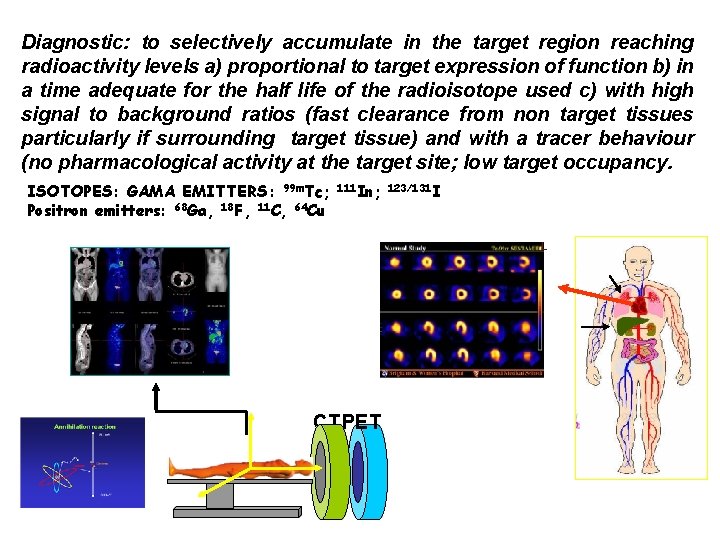
Diagnostic: to selectively accumulate in the target region reaching radioactivity levels a) proportional to target expression of function b) in a time adequate for the half life of the radioisotope used c) with high signal to background ratios (fast clearance from non target tissues particularly if surrounding target tissue) and with a tracer behaviour (no pharmacological activity at the target site; low target occupancy. ISOTOPES: GAMA EMITTERS: 99 m. Tc; Positron emitters: 68 Ga, 18 F, 11 C, 64 Cu 111 In; 123/131 I CT PET

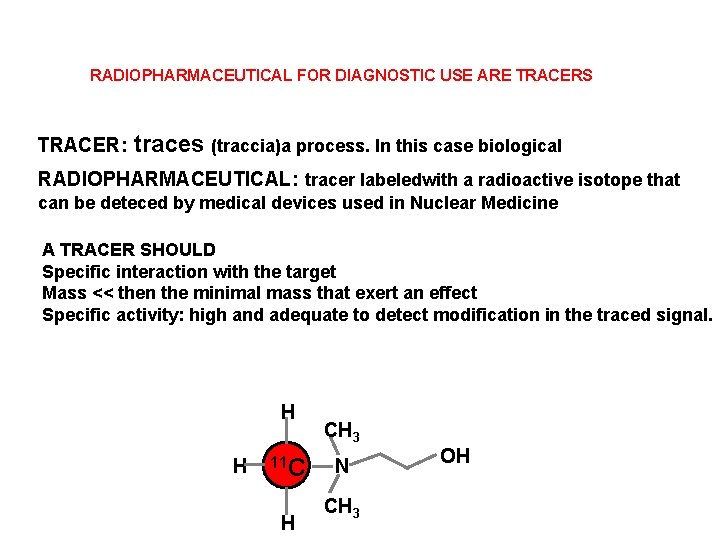
RADIOPHARMACEUTICAL FOR DIAGNOSTIC USE ARE TRACERS TRACER: traces (traccia)a process. In this case biological RADIOPHARMACEUTICAL: tracer labeledwith a radioactive isotope that can be deteced by medical devices used in Nuclear Medicine A TRACER SHOULD Specific interaction with the target Mass << then the minimal mass that exert an effect Specific activity: high and adequate to detect modification in the traced signal. H H 11 C H CH 3 N CH 3 OH

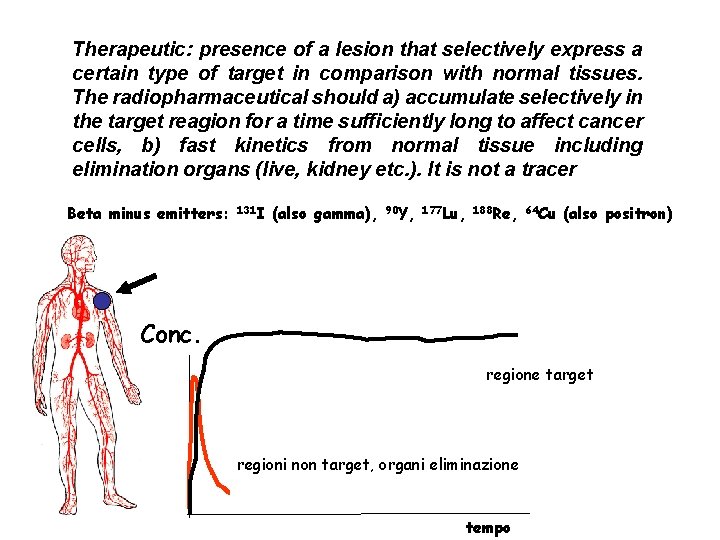
Therapeutic: presence of a lesion that selectively express a certain type of target in comparison with normal tissues. The radiopharmaceutical should a) accumulate selectively in the target reagion for a time sufficiently long to affect cancer cells, b) fast kinetics from normal tissue including elimination organs (live, kidney etc. ). It is not a tracer Beta minus emitters: 131 I (also gamma), 90 Y, 177 Lu, 188 Re, 64 Cu (also positron) Conc. regione target regioni non target, organi eliminazione tempo

Kinetics, aims UPTAKE AT TARGET SITE RETANTION PROPORTIONAL TO TARGET EXPRESSION/FUNCTION AND NOT OTHER VARIABLES CLEARANCE: FAST CLEARANCE FROM NON TARGET REGIONS LACK OF RADIOACTIVE METABOLITES AND IF PRESENT NOT AVAILABLE IN THE TARGET TISSUE

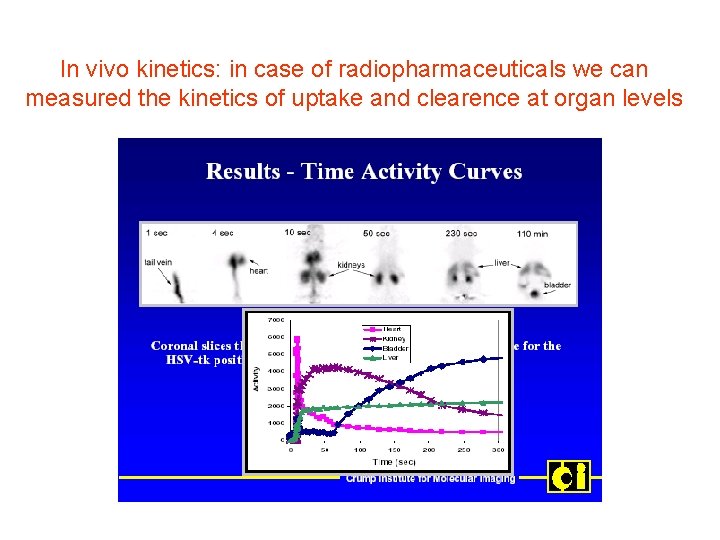
In vivo kinetics: in case of radiopharmaceuticals we can measured the kinetics of uptake and clearence at organ levels

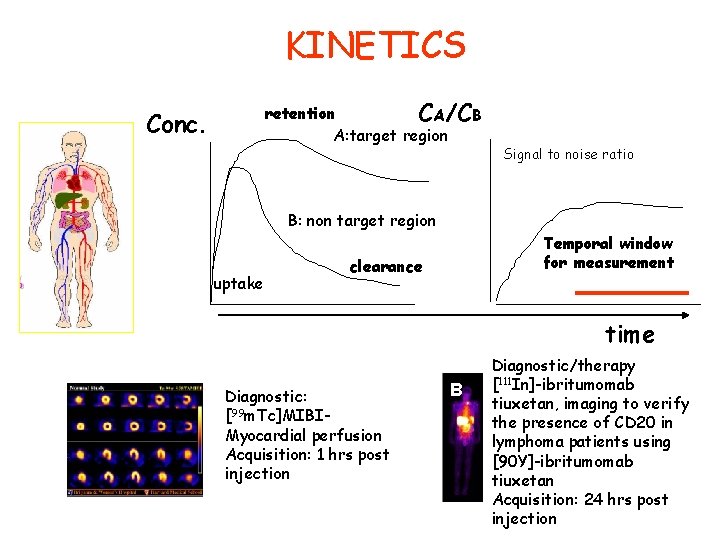
KINETICS retention CA/CB A: target region Conc. Signal to noise ratio B: non target region uptake Temporal window for measurement clearance time A Diagnostic: [99 m. Tc]MIBIMyocardial perfusion Acquisition: 1 hrs post injection B Diagnostic/therapy [111 In]-ibritumomab tiuxetan, imaging to verify the presence of CD 20 in lymphoma patients using [90 Y]-ibritumomab tiuxetan Acquisition: 24 hrs post injection

Upatake • • • Arival of the radiopharmaceutical in the target tissue. It is the major event in the first phase of distribution. It depends on: Organ Perfusion Diffusion across endotelial vascular membranes and eventually across BBB or cell membrane if the target is intracellular Plasma Protein binding or blood cell uptake

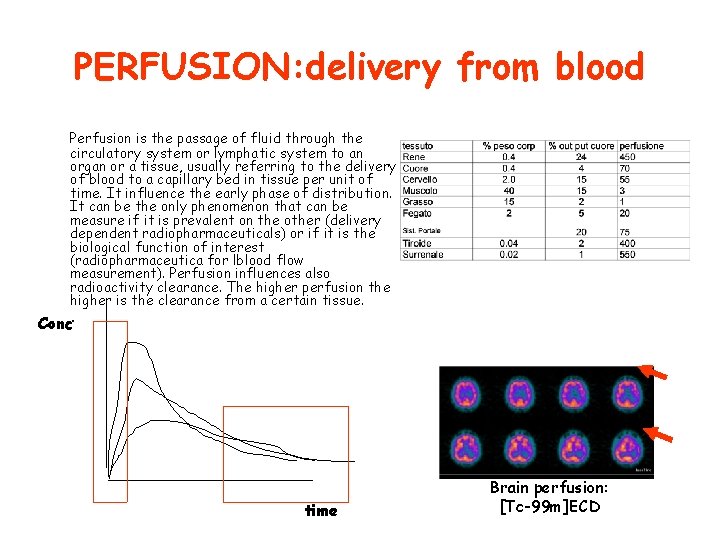
PERFUSION: delivery from blood Perfusion is the passage of fluid through the circulatory system or lymphatic system to an organ or a tissue, usually referring to the delivery of blood to a capillary bed in tissue per unit of time. It influence the early phase of distribution. It can be the only phenomenon that can be measure if it is prevalent on the other (delivery dependent radiopharmaceuticals) or if it is the biological function of interest (radiopharmaceutica for lblood flow measurement). Perfusion influences also radioactivity clearance. The higher perfusion the higher is the clearance from a certain tissue. Conc. time Brain perfusion: [Tc-99 m]ECD

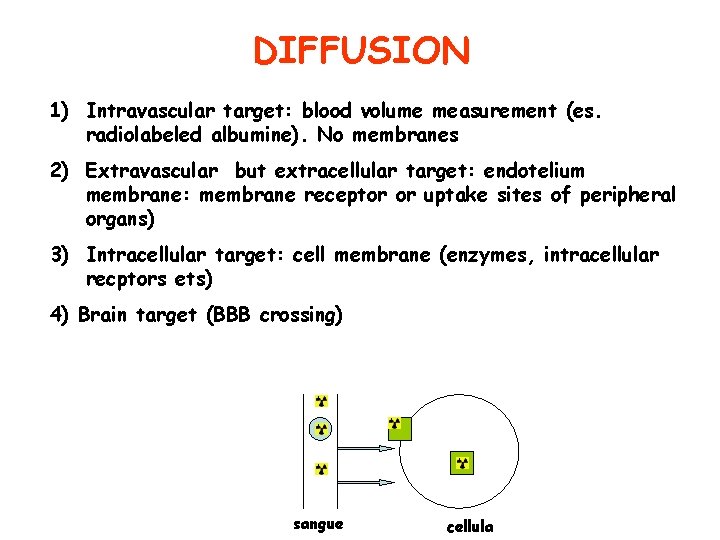
DIFFUSION 1) Intravascular target: blood volume measurement (es. radiolabeled albumine). No membranes 2) Extravascular but extracellular target: endotelium membrane: membrane receptor or uptake sites of peripheral organs) 3) Intracellular target: cell membrane (enzymes, intracellular recptors ets) 4) Brain target (BBB crossing) sangue cellula

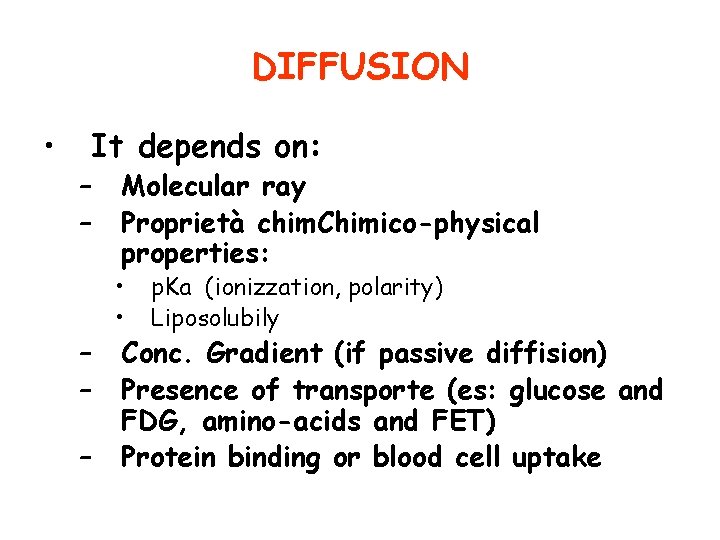
DIFFUSION • It depends on: – – – Molecular ray Proprietà chim. Chimico-physical properties: • • p. Ka (ionizzation, polarity) Liposolubily Conc. Gradient (if passive diffision) Presence of transporte (es: glucose and FDG, amino-acids and FET) Protein binding or blood cell uptake

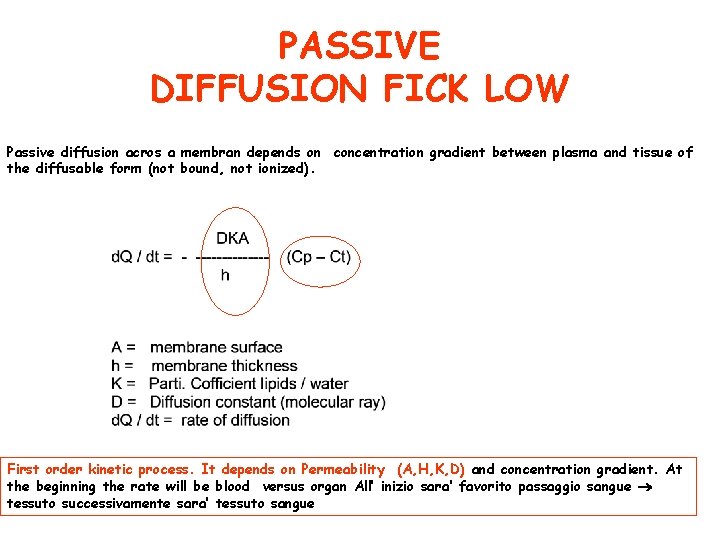
PASSIVE DIFFUSION FICK LOW Passive diffusion acros a membran depends on concentration gradient between plasma and tissue of the diffusable form (not bound, not ionized). First order kinetic process. It depends on Permeability (A, H, K, D) and concentration gradient. At the beginning the rate will be blood versus organ All’ inizio sara’ favorito passaggio sangue tessuto successivamente sara’ tessuto sangue

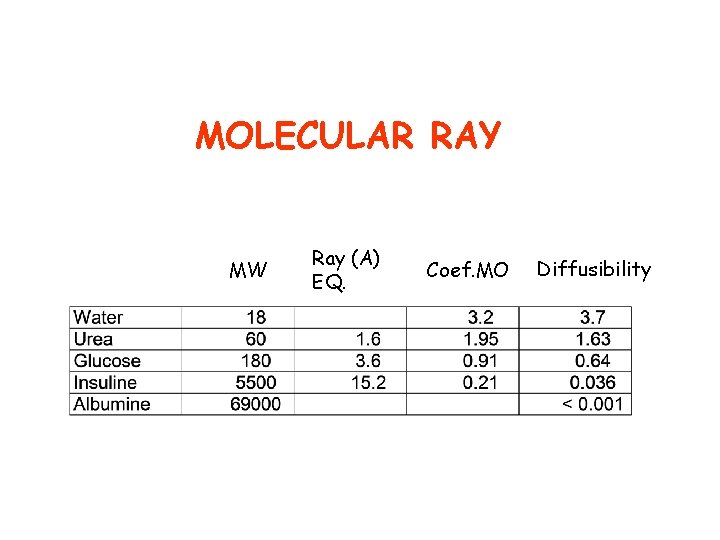
MOLECULAR RAY MW Ray (A) EQ. Coef. MO Diffusibility

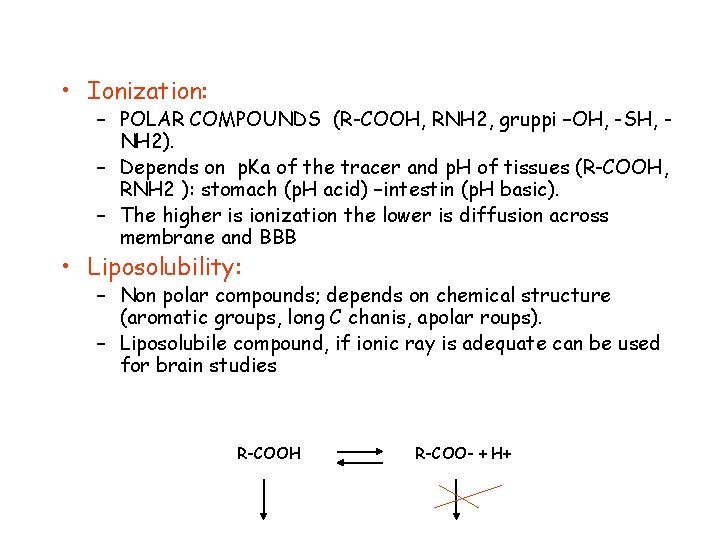
• Ionization: – POLAR COMPOUNDS (R-COOH, RNH 2, gruppi –OH, -SH, NH 2). – Depends on p. Ka of the tracer and p. H of tissues (R-COOH, RNH 2 ): stomach (p. H acid) –intestin (p. H basic). – The higher is ionization the lower is diffusion across membrane and BBB • Liposolubility: – Non polar compounds; depends on chemical structure (aromatic groups, long C chanis, apolar roups). – Liposolubile compound, if ionic ray is adequate can be used for brain studies R-COOH R-COO- + H+

Trasporter (FDG, FET, FLT, FDOPA etc. . . ) • Glucose (GLUT 1, 3 etc): [18 F]FDG • Aminoacid transporter: [11 C]Methionine, [18 F]fluoroethyl-tyrosine, [18 F]fluoro -tymidine • Choline tranporter: [11 C]Choline

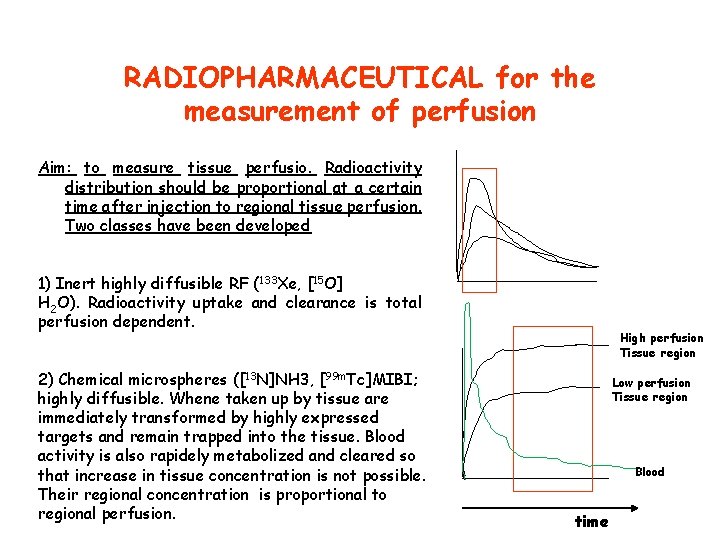
RADIOPHARMACEUTICAL for the measurement of perfusion Aim: to measure tissue perfusio. Radioactivity distribution should be proportional at a certain time after injection to regional tissue perfusion. Two classes have been developed 1) Inert highly diffusible RF (133 Xe, [15 O] H 2 O). Radioactivity uptake and clearance is total perfusion dependent. 2) Chemical microspheres ([13 N]NH 3, [99 m. Tc]MIBI; highly diffusible. Whene taken up by tissue are immediately transformed by highly expressed targets and remain trapped into the tissue. Blood activity is also rapidely metabolized and cleared so that increase in tissue concentration is not possible. Their regional concentration is proportional to regional perfusion. High perfusion Tissue region Low perfusion Tissue region Blood time

TARGETS OR PROCESSES • • Receptors Enzymes Transporter Antigens Reporter gene Proteins or cells Hypoxia hypoxia Microglia activation glycolisis

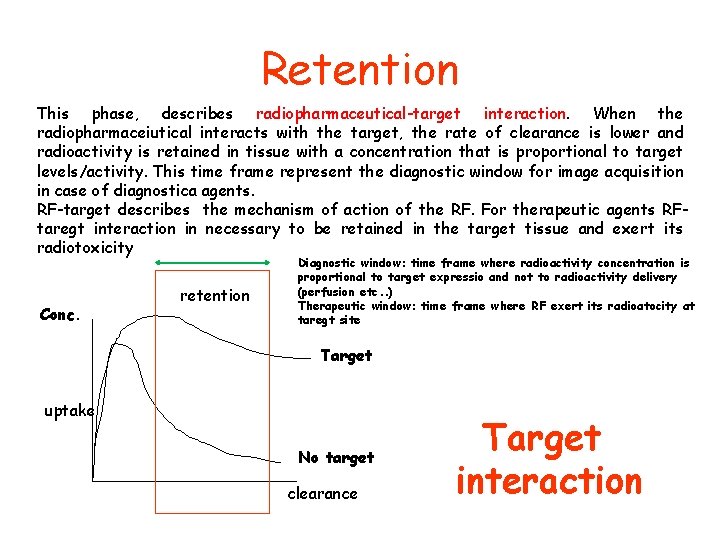
Retention This phase, describes radiopharmaceutical-target interaction. When the radiopharmaceiutical interacts with the target, the rate of clearance is lower and radioactivity is retained in tissue with a concentration that is proportional to target levels/activity. This time frame represent the diagnostic window for image acquisition in case of diagnostica agents. RF-target describes the mechanism of action of the RF. For therapeutic agents RFtaregt interaction in necessary to be retained in the target tissue and exert its radiotoxicity Conc. retention Diagnostic window: time frame where radioactivity concentration is proportional to target expressio and not to radioactivity delivery (perfusion etc. . ) Therapeutic window: time frame where RF exert its radioatocity at taregt site Target uptake No target clearance Target interaction

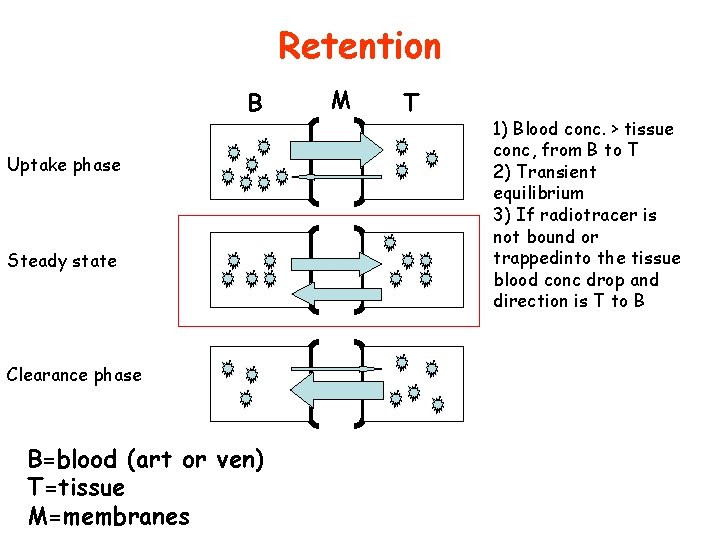
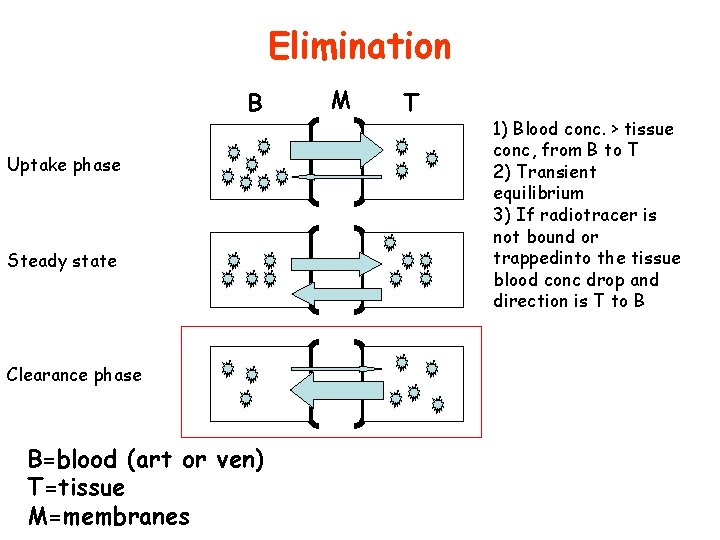
Retention B Uptake phase Steady state Clearance phase B=blood (art or ven) T=tissue M=membranes M T 1) Blood conc. > tissue conc, from B to T 2) Transient equilibrium 3) If radiotracer is not bound or trappedinto the tissue blood conc drop and direction is T to B

RETENTION • Biotransformation and trapping • Binding

Biotransformation and trapping • The radiopharmaceutical is transformed in a different chemical entity but manteining the radionuclide in its structure in general by an enzimatic reaction. The new chemical entity has a clearance rate from tissue >> slower than that of the original radiopharmaceutical or is trapped by tissue (clearance = 0) during measurement time frame. Tissue radioactivity increase with time reaching a maximum uptake that is proportional to the amount and activity of the target. The product is less soluble, bound to some intracellular component etc. . • Glycolisis: FDG-6 P (FDG=fluorodeoxyglucose) • Cell proliferation: FLT 6 P (FLT=fluoro-L-tymidine)
![18 Ffluorodeoxyglucose BBB tissue plasma F18FDG Cp F18FDGP No to Fructose 6 P [18 F]fluorodeoxyglucose BBB tissue plasma [F-18]FDG Cp F-18]FDG-P No to * Fructose -6 -P](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-45.jpg)
[18 F]fluorodeoxyglucose BBB tissue plasma [F-18]FDG Cp F-18]FDG-P No to * Fructose -6 -P GLUT HK Conc FDG+FDG 6 -P 60 min. FDG *Absence of the hydroxyl group in 2 precludes the isomerization from an aldehyde (glucose) to a ketone (fructose) chain and remein trapped inside the cell Time (min)

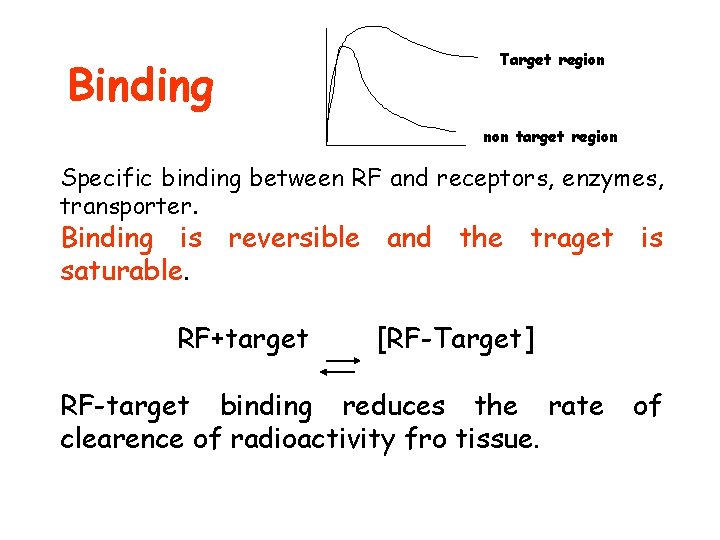
Binding Target region non target region Specific binding between RF and receptors, enzymes, transporter. Binding is reversible and the traget is saturable. RF+target [RF-Target] RF-target binding reduces the rate clearence of radioactivity fro tissue. of
![Binding Recettor or transporters 11 CRaclopride DAD 2 like rec 123 Binding • Recettor or transporters: • • [11 C]Raclopride- DA-D 2 like rec [123]](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-47.jpg)
Binding • Recettor or transporters: • • [11 C]Raclopride- DA-D 2 like rec [123] DATscan Dopamine transporter. [68 Ga]DOTATOC or DOTATATE. [68 Ga]PSMA • Proteins • [11 C]PIB – beta amyloid • New radiopharmaceuticals for tau

For a new Radiopharmacology it is crucial : a) Quality of preparation or production b) Efficacy: optimal acquisition, absence of radioactive metabolites at target site, time or therapeutic scheme (therapy) c) Safety: radioexposure and the absorbed dose (dosimetry). c

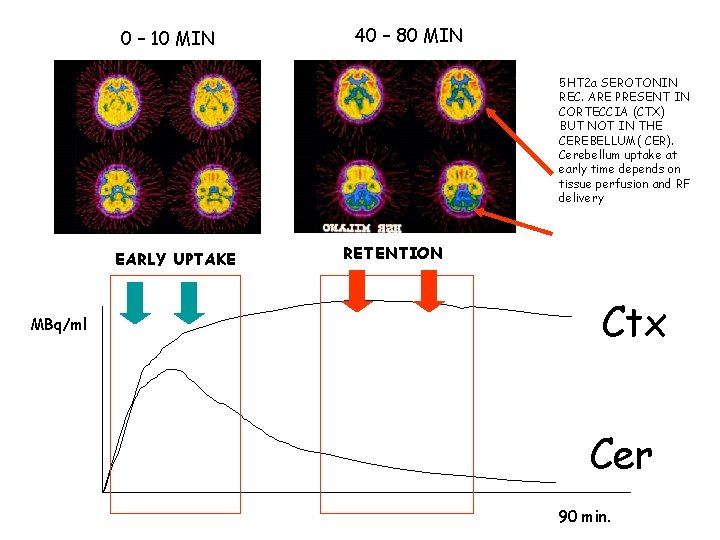
0 – 10 MIN 40 – 80 MIN 5 HT 2 a SEROTONIN REC. ARE PRESENT IN CORTECCIA (CTX) BUT NOT IN THE CEREBELLUM( CER). Cerebellum uptake at early time depends on tissue perfusion and RF delivery EARLY UPTAKE MBq/ml RETENTION Ctx Cer 90 min.

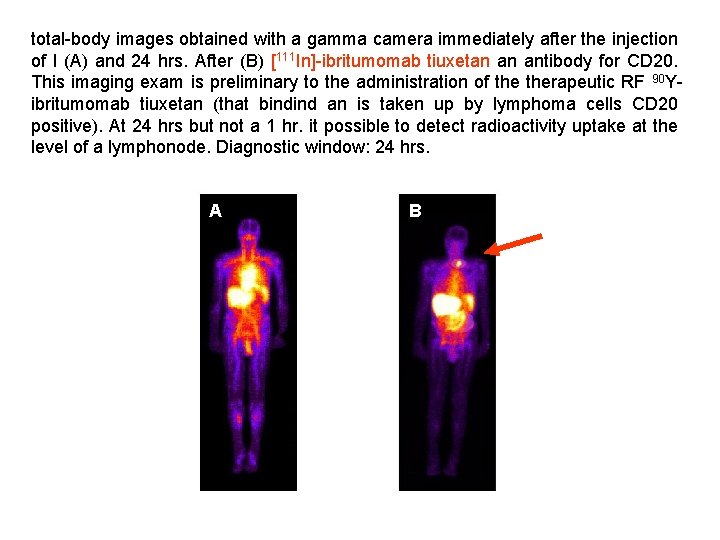
total-body images obtained with a gamma camera immediately after the injection of l (A) and 24 hrs. After (B) [111 In]-ibritumomab tiuxetan an antibody for CD 20. This imaging exam is preliminary to the administration of therapeutic RF 90 Yibritumomab tiuxetan (that bindind an is taken up by lymphoma cells CD 20 positive). At 24 hrs but not a 1 hr. it possible to detect radioactivity uptake at the level of a lymphonode. Diagnostic window: 24 hrs. A B

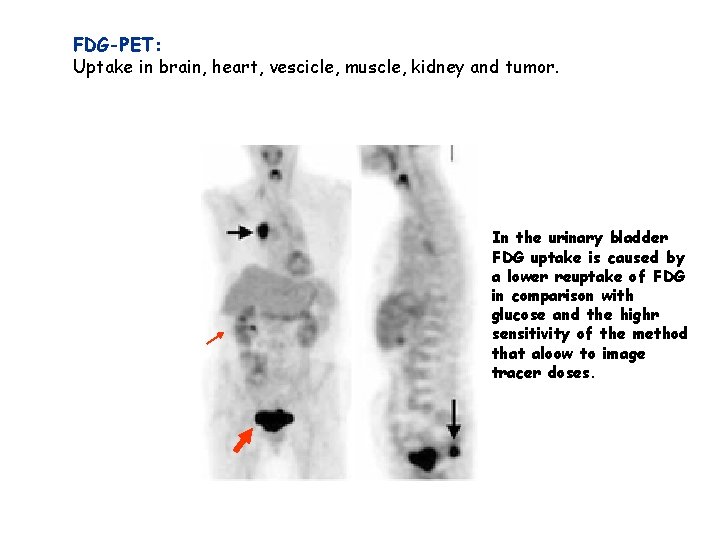
FDG-PET: Uptake in brain, heart, vescicle, muscle, kidney and tumor. In the urinary bladder FDG uptake is caused by a lower reuptake of FDG in comparison with glucose and the highr sensitivity of the method that aloow to image tracer doses.
![318 Ffluoro3deossitimidina 18 FFLT Cell proliferation marker Tymidine kinase I add a P group 3’-[18 F]fluoro-3’-deossitimidina ([18 F]FLT) Cell proliferation marker. Tymidine kinase I add a P group](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-52.jpg)
3’-[18 F]fluoro-3’-deossitimidina ([18 F]FLT) Cell proliferation marker. Tymidine kinase I add a P group to Tymidine and reduces its clearance from tumor cellsì Metabolism, eliminazion tumor Highly prolifera Melanoma

MECANISM OF ACTION INTERACTION WITH A BIOLOGICAL TARGET RELEVANT FOR DIAGNOSIS INTERACTION WITH A BIOLOGICAL TRAGET SELECTIVELY EXPRESSED IN THE TISSUE OF INTEREST (CANCER) WHERE THE RADIOPHARMACEUTRICALS CARRY THE RADIONIUCLIDE TO KILLS CANCER CELLS

Elimination of radiopharmaceuticals from the body Kidney Excretion (hydrophilic compounds) Biliary Excretion (lypophilic compounds) In general before to be excreted the molecule is modified by metabolic phase I or phase II processes that occur in liver. In case of a radiopharmaceutical clerance is caused also by the radioactive decay of the radionuclide.

Elimination B Uptake phase Steady state Clearance phase B=blood (art or ven) T=tissue M=membranes M T 1) Blood conc. > tissue conc, from B to T 2) Transient equilibrium 3) If radiotracer is not bound or trappedinto the tissue blood conc drop and direction is T to B

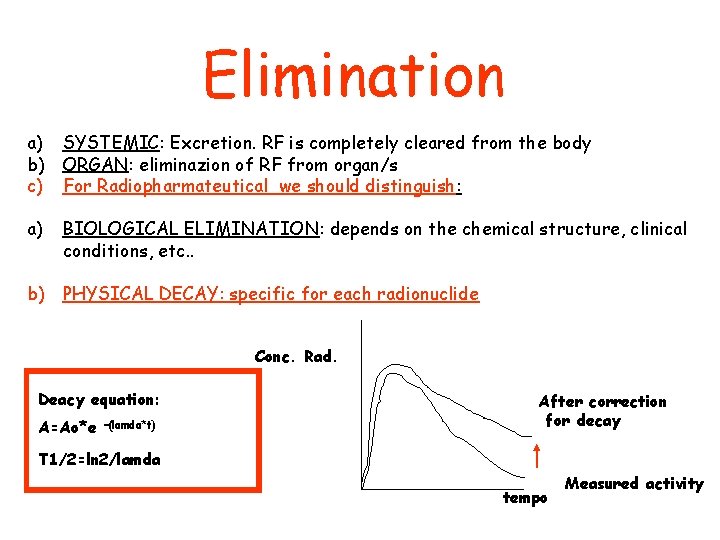
Elimination a) SYSTEMIC: Excretion. RF is completely cleared from the body b) ORGAN: eliminazion of RF from organ/s c) For Radiopharmateutical we should distinguish: a) BIOLOGICAL ELIMINATION: depends on the chemical structure, clinical conditions, etc. . b) PHYSICAL DECAY: specific for each radionuclide Conc. Rad. Deacy equation: A=Ao*e –(lamda*t) After correction for decay T 1/2=ln 2/lamda tempo Measured activity

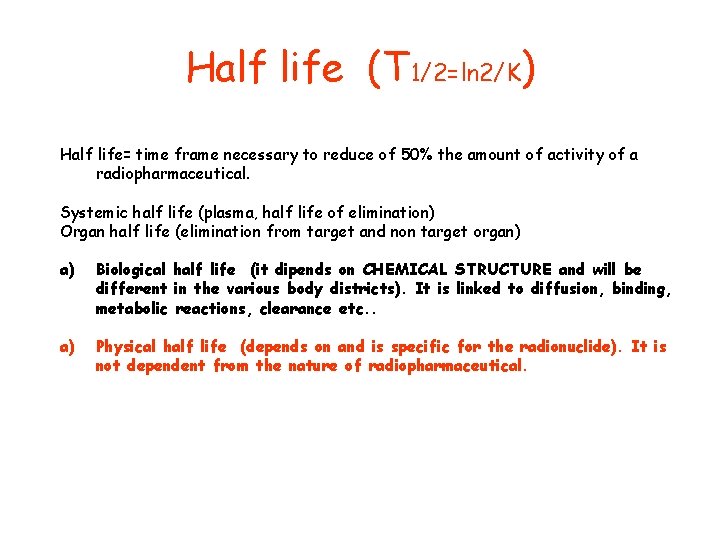
Half life (T 1/2=ln 2/K) Half life= time frame necessary to reduce of 50% the amount of activity of a radiopharmaceutical. Systemic half life (plasma, half life of elimination) Organ half life (elimination from target and non target organ) a) Biological half life (it dipends on CHEMICAL STRUCTURE and will be different in the various body districts). It is linked to diffusion, binding, metabolic reactions, clearance etc. . a) Physical half life (depends on and is specific for the radionuclide). It is not dependent from the nature of radiopharmaceutical.

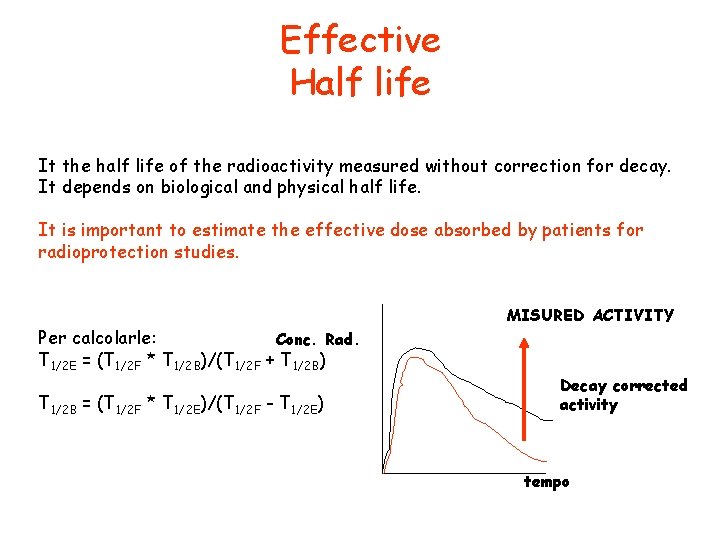
Effective Half life It the half life of the radioactivity measured without correction for decay. It depends on biological and physical half life. It is important to estimate the effective dose absorbed by patients for radioprotection studies. Per calcolarle: Conc. Rad. T 1/2 E = (T 1/2 F * T 1/2 B)/(T 1/2 F + T 1/2 B) T 1/2 B = (T 1/2 F * T 1/2 E)/(T 1/2 F - T 1/2 E) MISURED ACTIVITY Decay corrected activity tempo

Half life For short half life radionuclides : If physical T 1/2 is << of biological T 1/2: Dosimetry: only phycal half life should be considered. Diagnostic: the optimal window of measurement should be at a time adequate for the short half life of the isotope.

Tracer development Selection Cold ligand developmen Target validation Radiochemical development Radiochemistry In vitro evaluation Biological development In vivo Small animals Target selection Small animal model of diseases DOSIMETRY Normal volunteers Human being Clinical studies in patients
![18 FFDG GLUCOSE ANALOGUE n Transported as glucose with GLUx transporter n Phosphorilated in [18 F]FDG: GLUCOSE ANALOGUE n. Transported as glucose with GLUx transporter n. Phosphorilated in](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-61.jpg)
[18 F]FDG: GLUCOSE ANALOGUE n. Transported as glucose with GLUx transporter n. Phosphorilated in 6 by HK [18 F]FDG-6 -P n Trapped inside the cell Intracellular uptake is proportional to GLUT levels on the membrane and HK activity inside the cell. Appplications: Neurology Oncology Inflammation
![18 FFDG as an example Deoxyglucose is STOPED AS 6 PHOSPHATE EXOSES [18 F]FDG as an example Deoxyglucose is STOPED AS 6 PHOSPHATE EXOSES](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-62.jpg)
[18 F]FDG as an example Deoxyglucose is STOPED AS 6 PHOSPHATE EXOSES
![2 F18fluoro2 deoxyDGlucose F18FDG GLUT FDG blood Brain retention of FDG is dependent on 2 -[F-18]fluoro-2 -deoxy-D-Glucose [F-18]FDG) GLUT FDG blood Brain retention of FDG is dependent on](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-63.jpg)
2 -[F-18]fluoro-2 -deoxy-D-Glucose [F-18]FDG) GLUT FDG blood Brain retention of FDG is dependent on hexokinase activity HK FDG-6 P brain Fructose-6 P

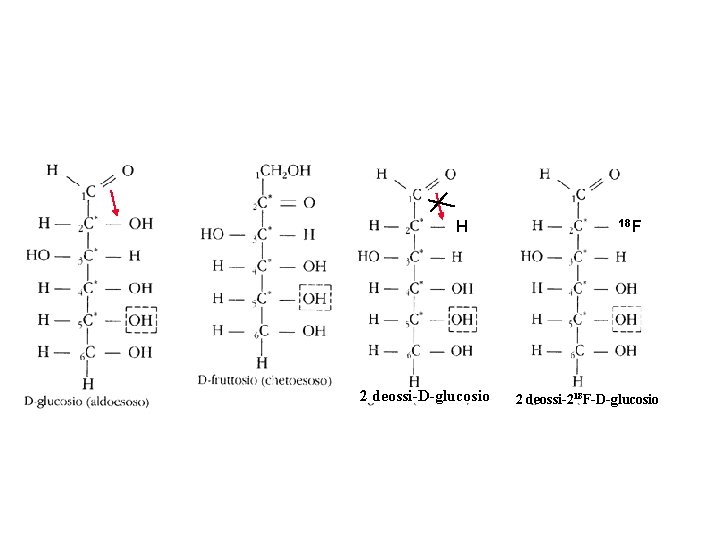
Isomerizzation from aldehyde to ketone not possible because of the lack of the hydroxil in 2 position H 2 deossi-D-glucosio 18 F 2 deossi-218 F-D-glucosio
![18 FFDG humans Fasting conditions [18 F]FDG humans Fasting conditions](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-65.jpg)
[18 F]FDG humans Fasting conditions

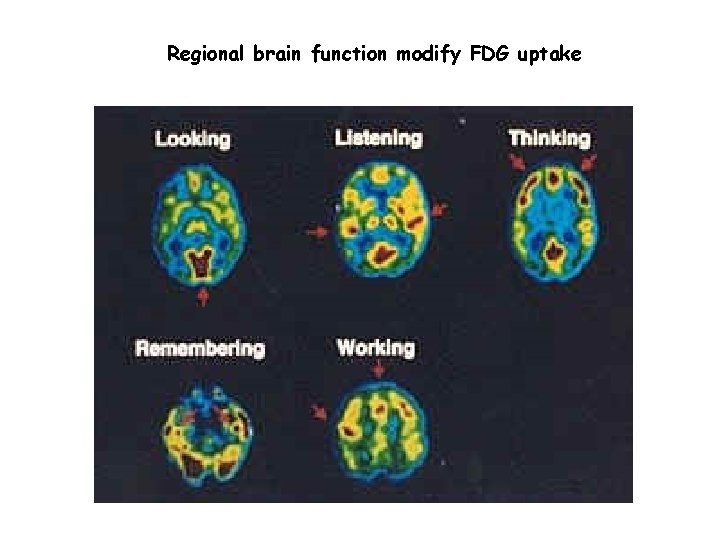
Regional brain function modify FDG uptake

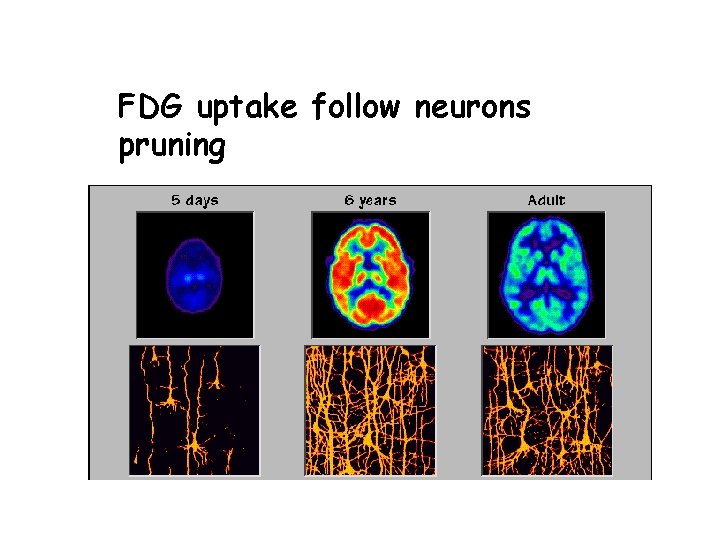
FDG uptake follow neurons pruning

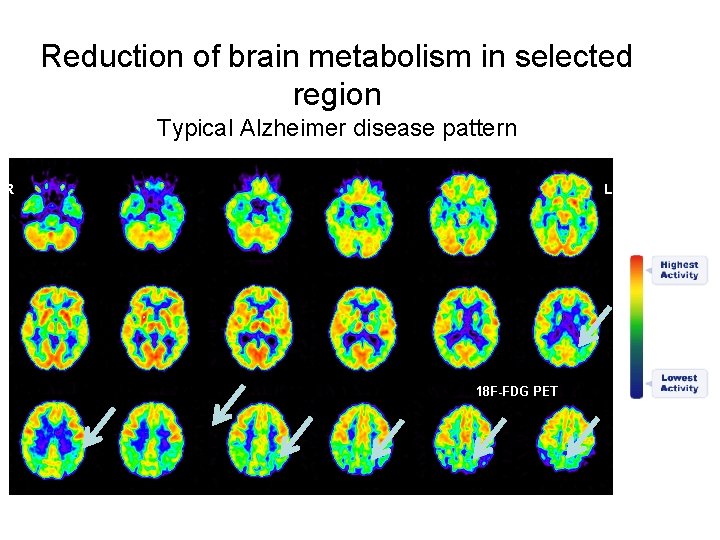
Reduction of brain metabolism in selected region Typical Alzheimer disease pattern R L
![Why 18 FFDGi n cancer Why [18 F]FDGi n cancer?](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-69.jpg)
Why [18 F]FDGi n cancer?
![Why 18 FFDGi n cancer n Higher glycolisis Warburg effect increase in glycolis in Why [18 F]FDGi n cancer? n Higher glycolisis (Warburg effect: increase in glycolis in](https://slidetodoc.com/presentation_image/44d1f656b8ac114ab5c9b8681424f2b9/image-70.jpg)
Why [18 F]FDGi n cancer? n Higher glycolisis (Warburg effect: increase in glycolis in presence of oxygen) n Increased uptake phosphorilation of glucose n Tight correlation between FDG uptake and cell viability and


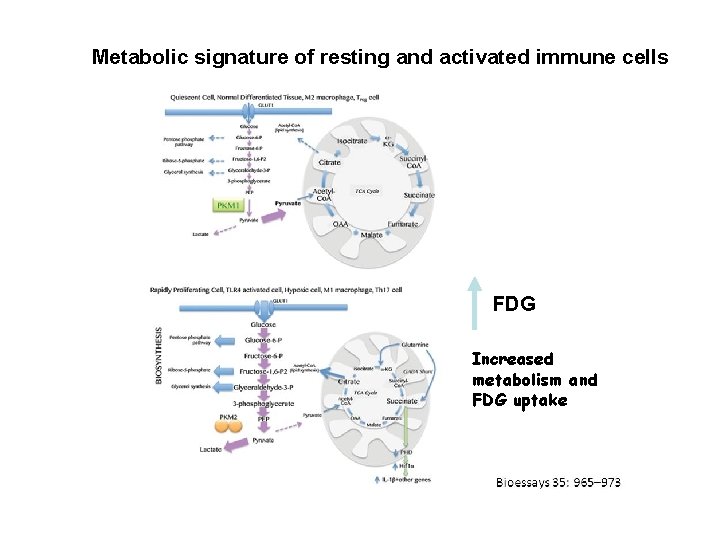
Metabolic signature of resting and activated immune cells FDG Increased metabolism and FDG uptake

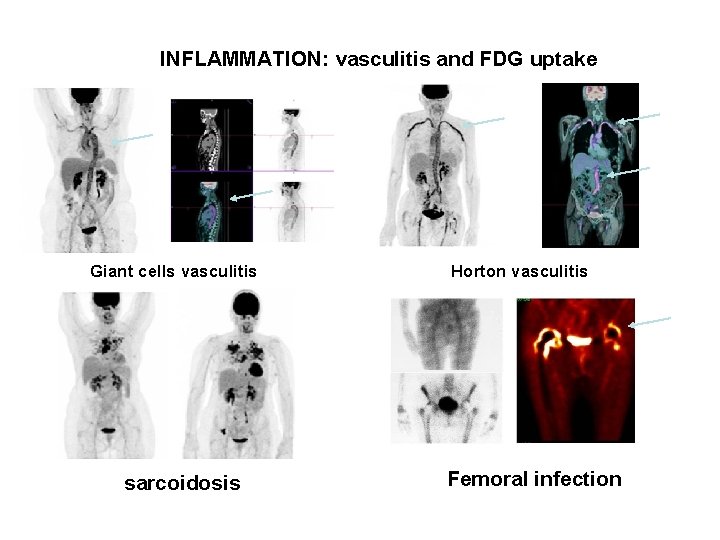
INFLAMMATION: vasculitis and FDG uptake Giant cells vasculitis Horton vasculitis sarcoidosis Femoral infection