Diagnostic criteria A full 10 years after Rome








- Slides: 43





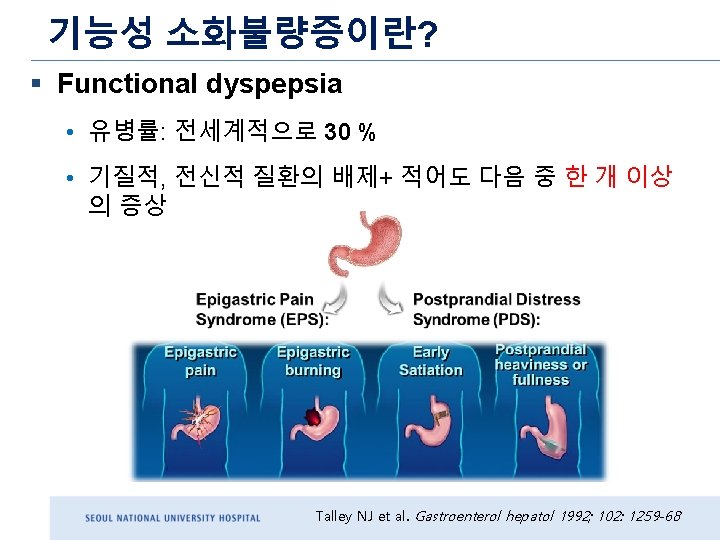
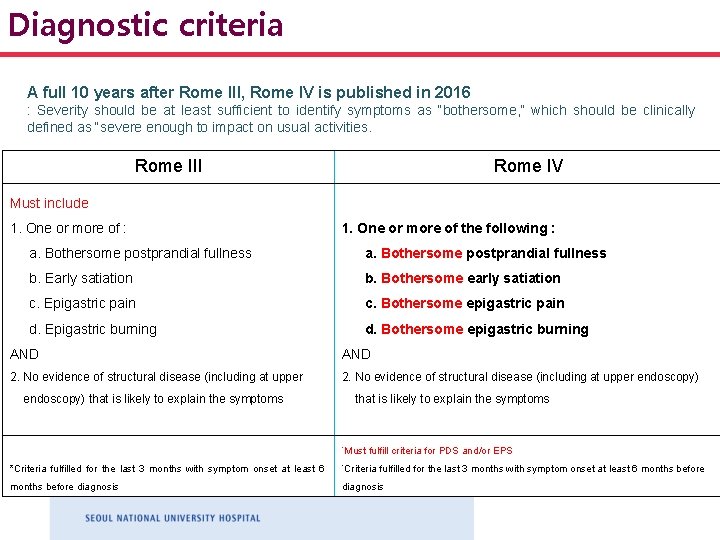
Diagnostic criteria A full 10 years after Rome III, Rome IV is published in 2016 : Severity should be at least sufficient to identify symptoms as “bothersome, ” which should be clinically defined as “severe enough to impact on usual activities. Rome III Rome IV Must include 1. One or more of : 1. One or more of the following : a. Bothersome postprandial fullness b. Early satiation b. Bothersome early satiation c. Epigastric pain c. Bothersome epigastric pain d. Epigastric burning d. Bothersome epigastric burning AND 2. No evidence of structural disease (including at upper endoscopy) that is likely to explain the symptoms *Must fulfill criteria for PDS and/or EPS *Criteria fulfilled for the last 3 months with symptom onset at least 6 months before diagnosis

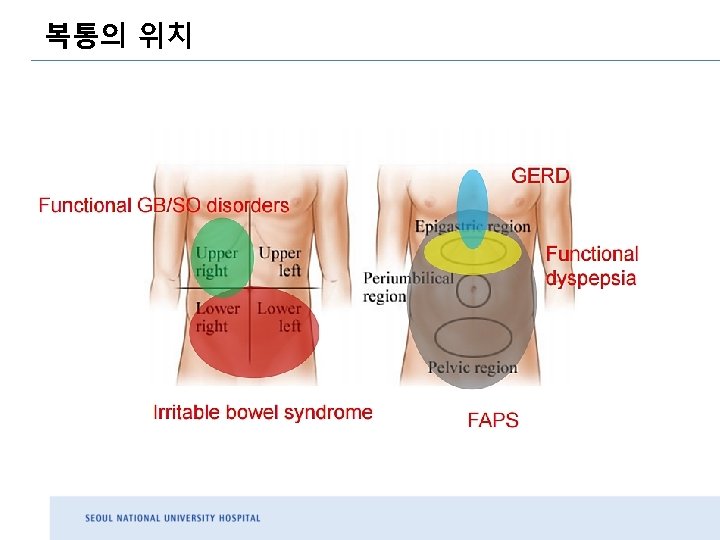
ROME III ROME IV 1. Upper abdominal bloating or postprandial nausea or excessive belching can be present 1. Postprandial epigastric pain or burning, epigastric bloating excessive belching, and nausea can also be present 2. Vomiting warrants consideration of another disorder 3. Heartburn is not a dyspeptic symptom but may often co-exist 4. Symptoms that are relieved by evacuation of feces or gas should generally not be considered as part of dyspepsia 5. GERD and IBS may co-exist with PDS 2. Epigastric pain syndrome may coexist

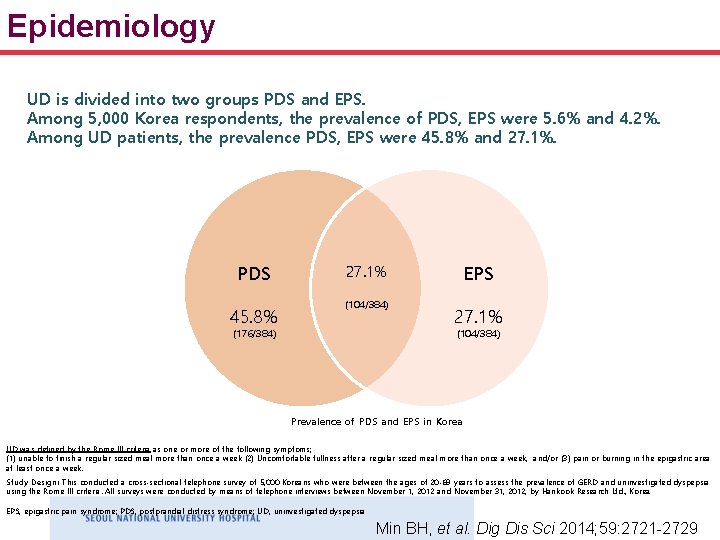
Epidemiology UD is divided into two groups PDS and EPS. Among 5, 000 Korea respondents, the prevalence of PDS, EPS were 5. 6% and 4. 2%. Among UD patients, the prevalence PDS, EPS were 45. 8% and 27. 1%. PDS 45. 8% 27. 1% (104/384) (176/384) EPS 27. 1% (104/384) Prevalence of PDS and EPS in Korea UD was defined by the Rome III criteria as one or more of the following symptoms; (1) unable to finish a regular sized meal more than once a week (2) Uncomfortable fullness after a regular sized meal more than once a week, and/or (3) pain or burning in the epigastric area at least once a week. Study Design: This conducted a cross-sectional telephone survey of 5, 000 Koreans who were between the ages of 20 -69 years to assess the prevalence of GERD and uninvestigated dyspepsia using the Rome III criteria. All surveys were conducted by means of telephone interviews between November 1, 2012 and November 31, 2012, by Hankook Research Ltd. , Korea. EPS, epigastric pain syndrome; PDS, postprandial distress syndrome; UD, uninvestigated dyspepsia Min BH, et al. Dig Dis Sci 2014; 59: 2721 -2729

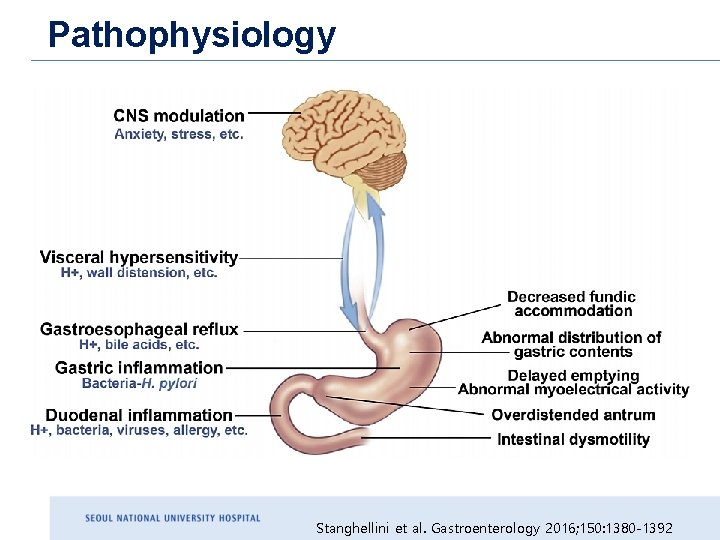
Pathophysiology Stanghellini et al. Gastroenterology 2016; 150: 1380 -1392

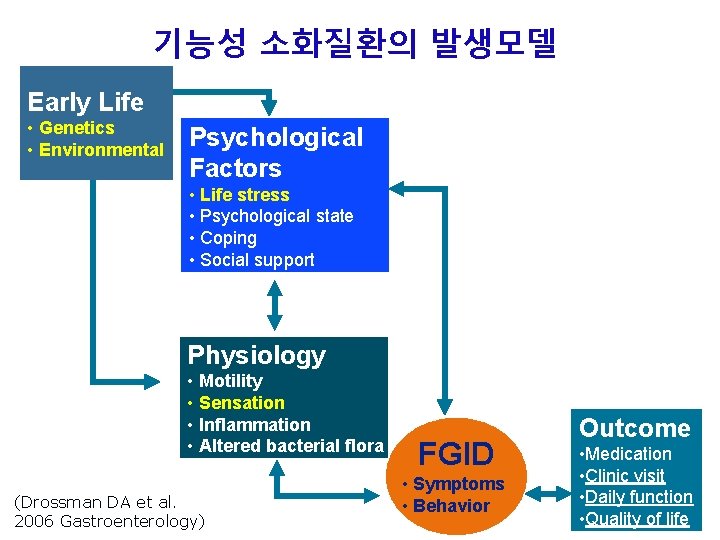
기능성 소화질환의 발생모델 Early Life • Genetics • Environmental Psychological Factors • Life stress • Psychological state • Coping • Social support Brain CNS Gut ENS Physiology • Motility • Sensation • Inflammation • Altered bacterial flora (Drossman DA et al. 2006 Gastroenterology) FGID • Symptoms • Behavior Outcome • Medication • Clinic visit • Daily function • Quality of life

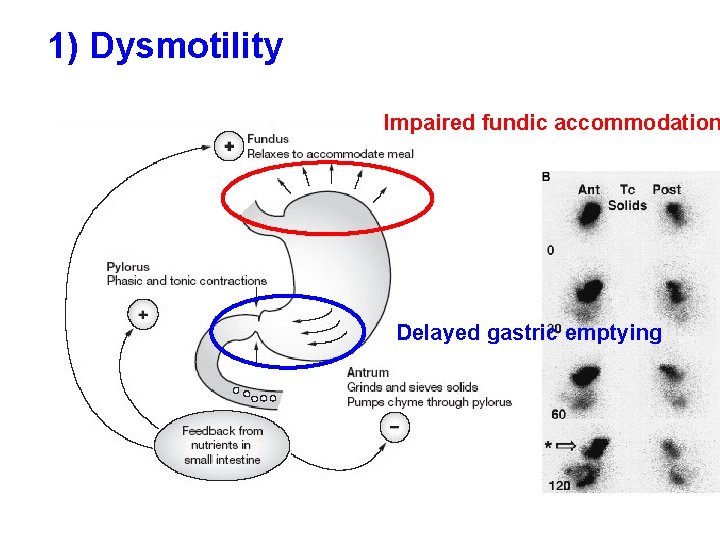
1) Dysmotility Impaired fundic accommodation Delayed gastric emptying


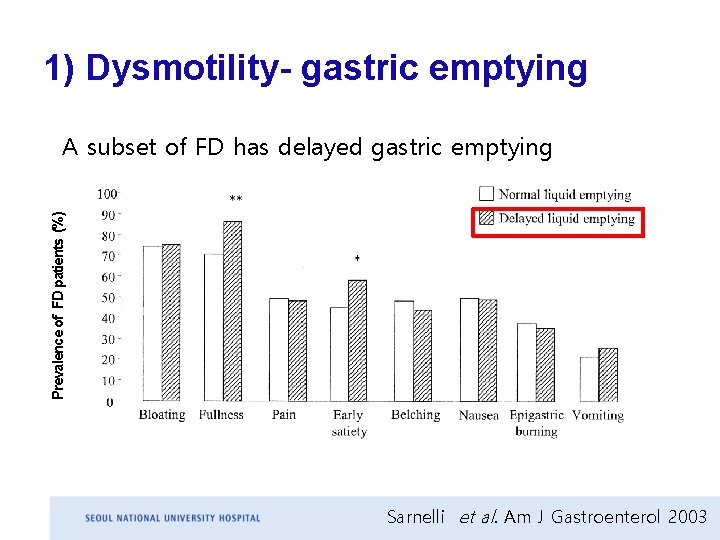
1) Dysmotility- gastric emptying Prevalence of FD patients (%) A subset of FD has delayed gastric emptying Sarnelli et al. Am J Gastroenterol 2003

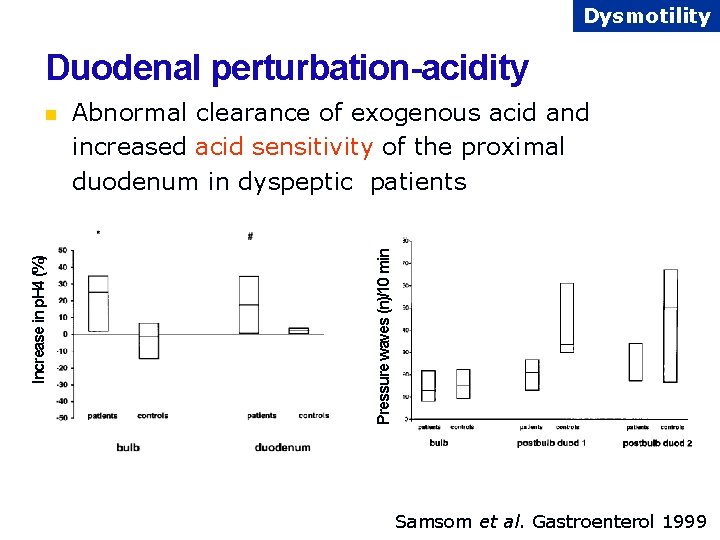
Dysmotility Duodenal perturbation-acidity Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients Pressure waves (n)/10 min Increase in p. H 4 (%) n Samsom et al. Gastroenterol 1999

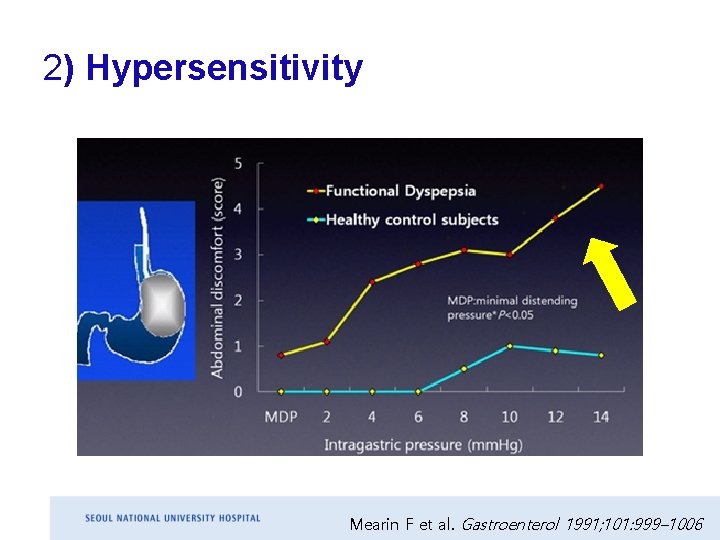
2) Hypersensitivity Mearin F et al. Gastroenterol 1991; 101: 999– 1006

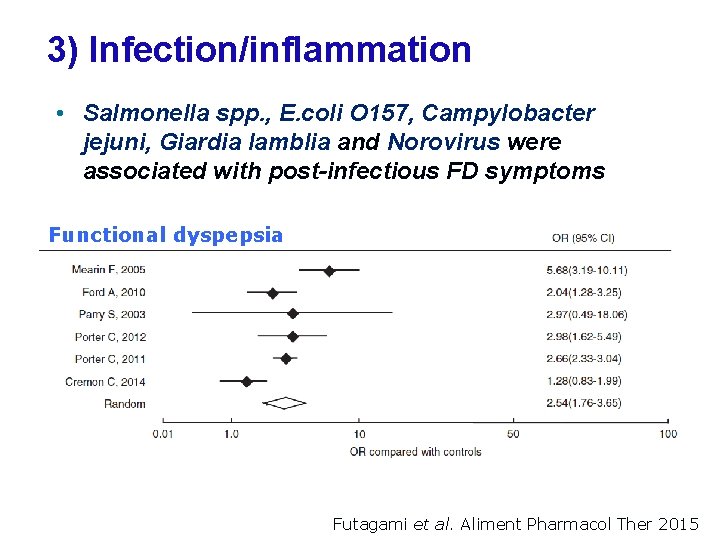
3) Infection/inflammation • Salmonella spp. , E. coli O 157, Campylobacter jejuni, Giardia lamblia and Norovirus were associated with post-infectious FD symptoms Functional dyspepsia Futagami et al. Aliment Pharmacol Ther 2015

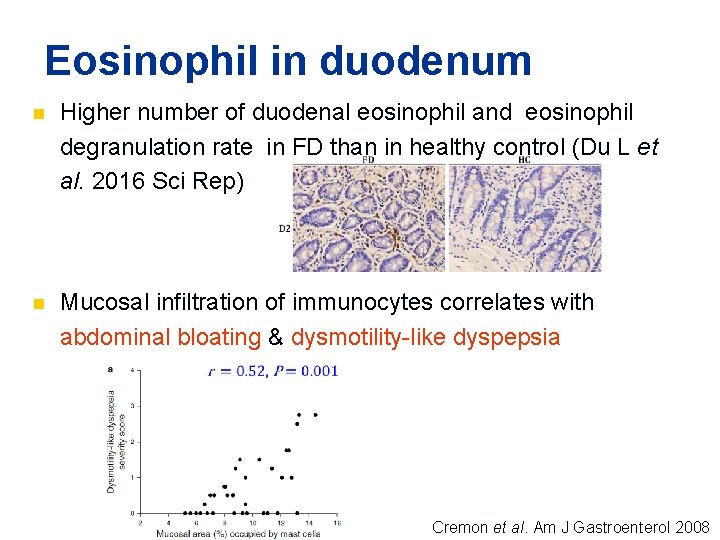
Eosinophil in duodenum n Higher number of duodenal eosinophil and eosinophil degranulation rate in FD than in healthy control (Du L et al. 2016 Sci Rep) n Mucosal infiltration of immunocytes correlates with abdominal bloating & dysmotility-like dyspepsia Cremon et al. Am J Gastroenterol 2008

4) H. pylori Budzyński et al. 2014 WJG

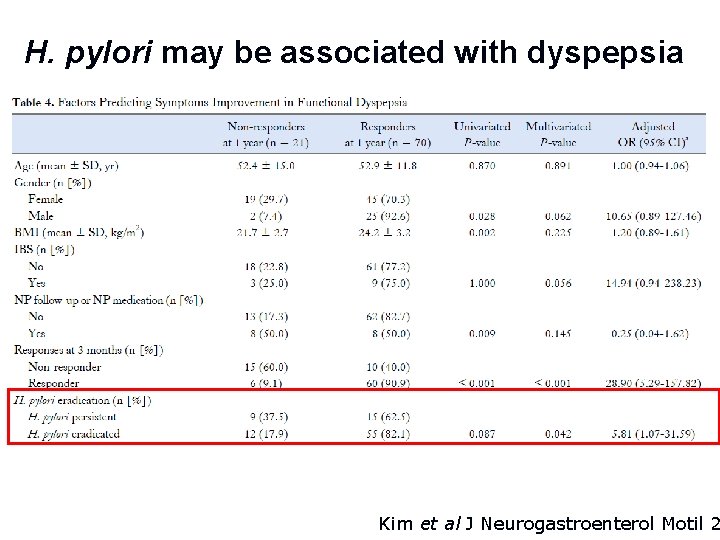
H. pylori may be associated with dyspepsia Kim et al J Neurogastroenterol Motil 2

5) Psychopathological factor • Concomitant psychiatric disorders in FD is very frequent (Tse et al. 2010 JNM)

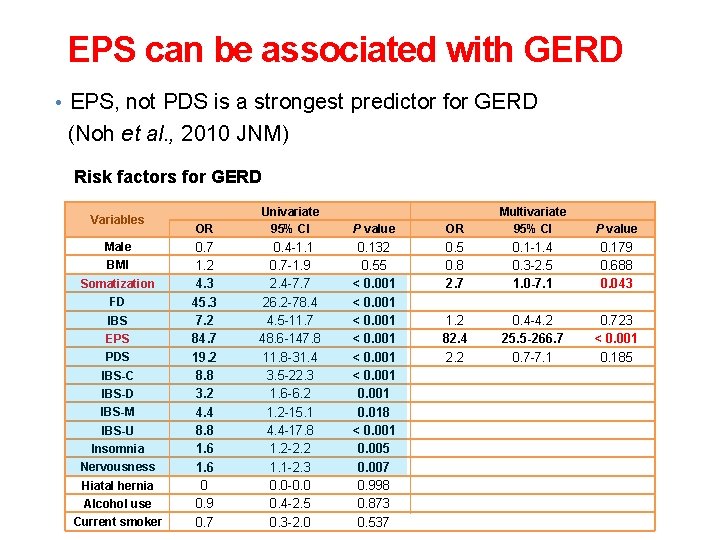
EPS can be associated with GERD • EPS, not PDS is a strongest predictor for GERD (Noh et al. , 2010 JNM) Risk factors for GERD Variables Male BMI Somatization FD IBS EPS PDS IBS-C IBS-D IBS-M IBS-U Insomnia Nervousness Hiatal hernia Alcohol use Current smoker OR Univariate 95% CI P value OR Multivariate 95% CI P value 0. 7 1. 2 4. 3 45. 3 7. 2 84. 7 19. 2 8. 8 3. 2 4. 4 8. 8 1. 6 0 0. 9 0. 7 0. 4 -1. 1 0. 7 -1. 9 2. 4 -7. 7 26. 2 -78. 4 4. 5 -11. 7 48. 6 -147. 8 11. 8 -31. 4 3. 5 -22. 3 1. 6 -6. 2 1. 2 -15. 1 4. 4 -17. 8 1. 2 -2. 2 1. 1 -2. 3 0. 0 -0. 0 0. 4 -2. 5 0. 3 -2. 0 0. 132 0. 55 < 0. 001 0. 018 < 0. 001 0. 005 0. 007 0. 998 0. 873 0. 537 0. 5 0. 8 2. 7 0. 1 -1. 4 0. 3 -2. 5 1. 0 -7. 1 0. 179 0. 688 0. 043 1. 2 82. 4 2. 2 0. 4 -4. 2 25. 5 -266. 7 0. 7 -7. 1 0. 723 < 0. 001 0. 185

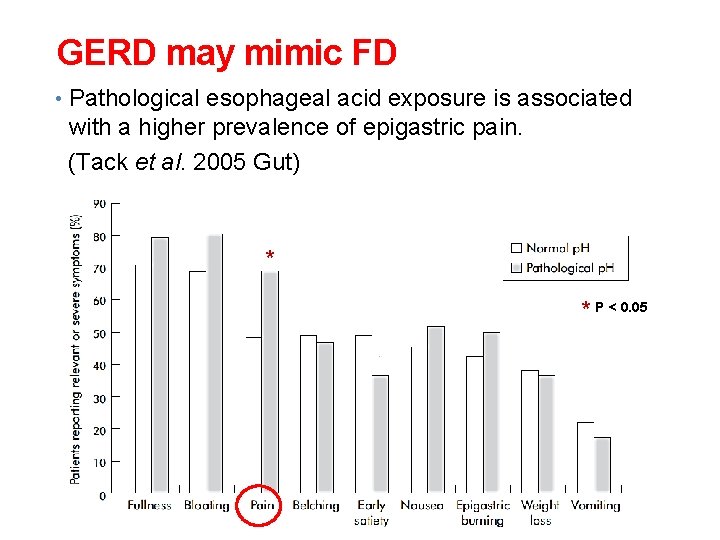
GERD may mimic FD • Pathological esophageal acid exposure is associated with a higher prevalence of epigastric pain. (Tack et al. 2005 Gut) * * P < 0. 05

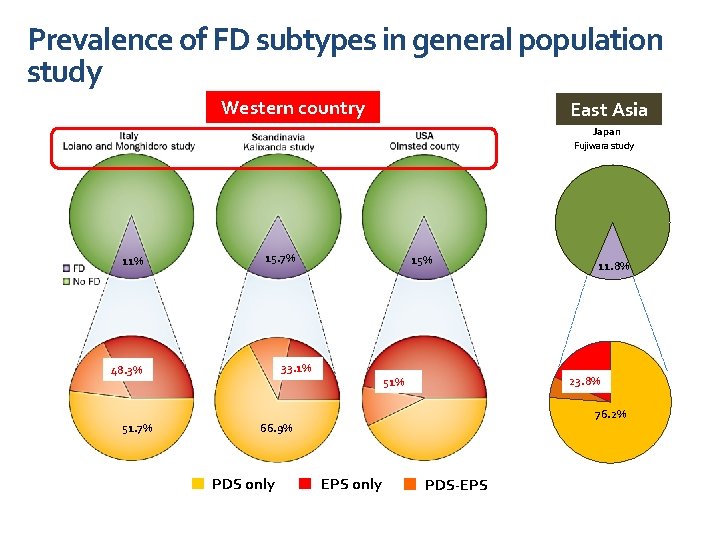
Prevalence of FD subtypes in general population study Western country East Asia Japan Fujiwara study 11% 15. 7% 33. 1% 48. 3% 51. 7% 15% 23. 8% 51% 76. 2% 66. 9% PDS only 11. 8% EPS only PDS-EPS


치료 1) Diet 2) Pharmacologic therapy

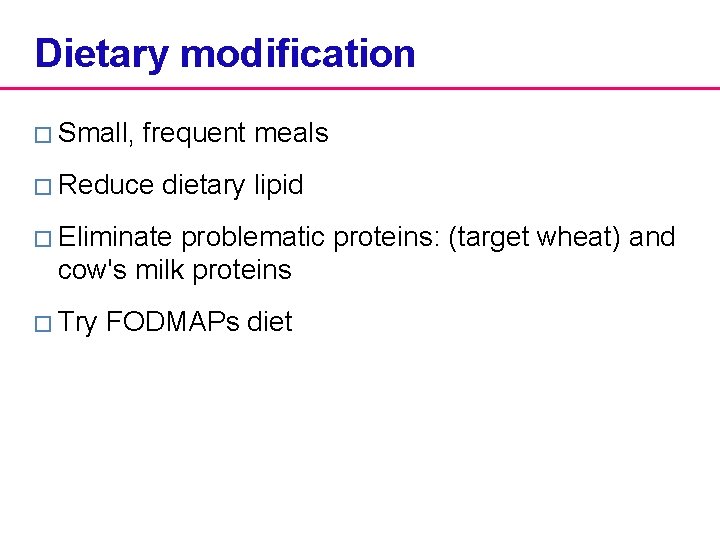
Dietary modification � Small, frequent meals � Reduce dietary lipid � Eliminate problematic proteins: (target wheat) and cow's milk proteins � Try FODMAPs diet



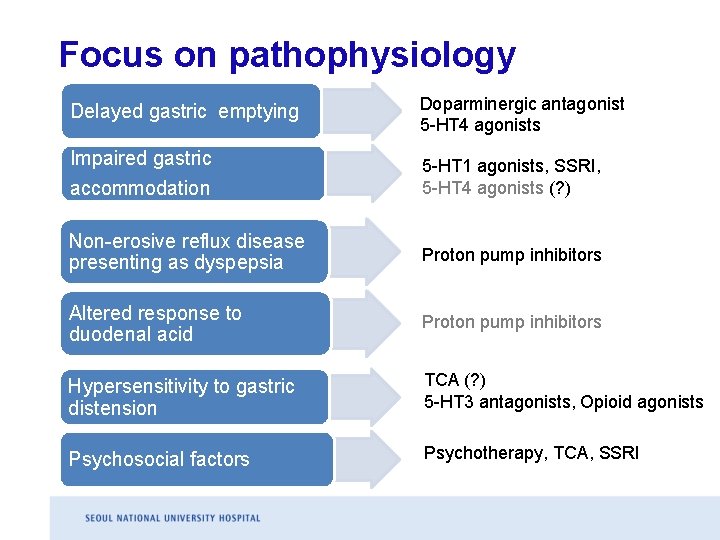
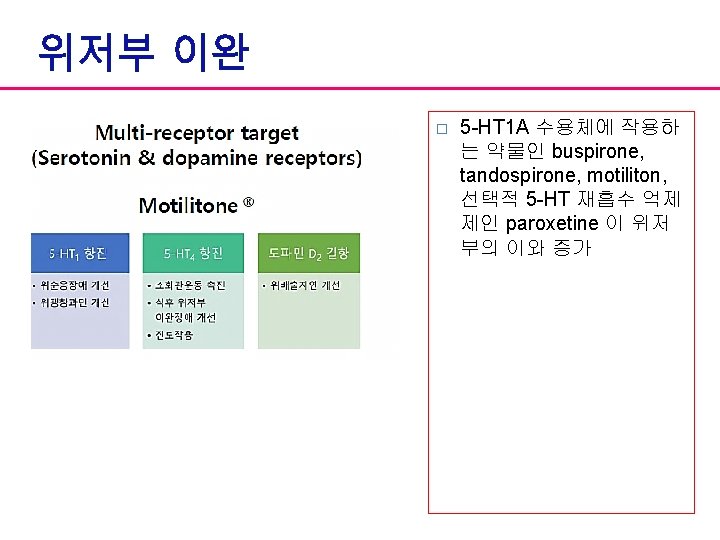
Focus on pathophysiology Delayed gastric emptying Doparminergic antagonist 5 -HT 4 agonists Impaired gastric accommodation 5 -HT 1 agonists, SSRI, 5 -HT 4 agonists (? ) Non-erosive reflux disease presenting as dyspepsia Proton pump inhibitors Altered response to duodenal acid Proton pump inhibitors Hypersensitivity to gastric distension TCA (? ) 5 -HT 3 antagonists, Opioid agonists Psychosocial factors Psychotherapy, TCA, SSRI

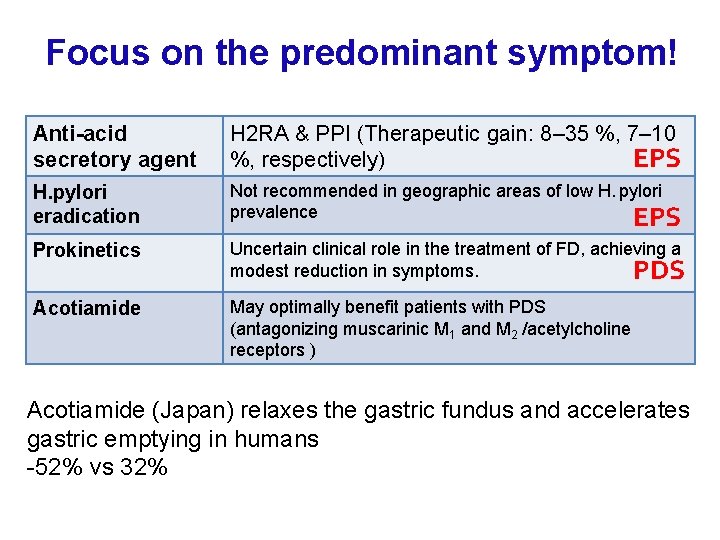
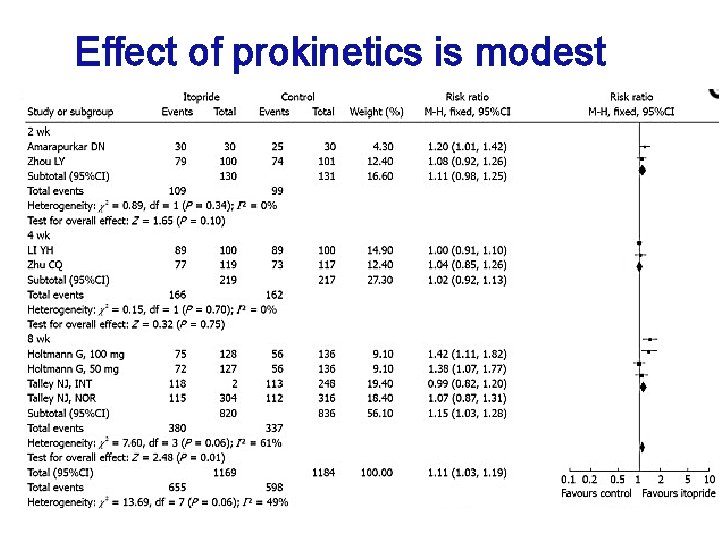
Focus on the predominant symptom! Anti-acid secretory agent H 2 RA & PPI (Therapeutic gain: 8– 35 %, 7– 10 EPS %, respectively) H. pylori eradication Not recommended in geographic areas of low H. pylori prevalence EPS Prokinetics Uncertain clinical role in the treatment of FD, achieving a modest reduction in symptoms. PDS Acotiamide May optimally benefit patients with PDS (antagonizing muscarinic M 1 and M 2 /acetylcholine receptors ) Acotiamide (Japan) relaxes the gastric fundus and accelerates gastric emptying in humans -52% vs 32%

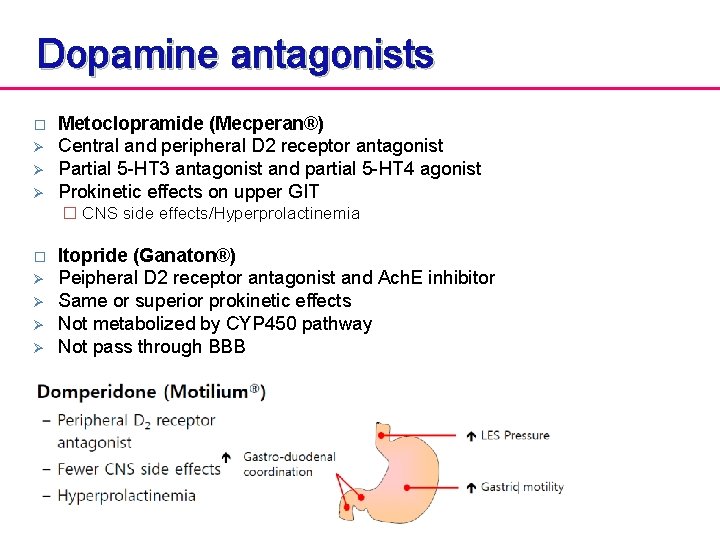
Dopamine antagonists � Ø Ø Ø Metoclopramide (Mecperan®) Central and peripheral D 2 receptor antagonist Partial 5 -HT 3 antagonist and partial 5 -HT 4 agonist Prokinetic effects on upper GIT � CNS side effects/Hyperprolactinemia � Ø Ø Itopride (Ganaton®) Peipheral D 2 receptor antagonist and Ach. E inhibitor Same or superior prokinetic effects Not metabolized by CYP 450 pathway Not pass through BBB

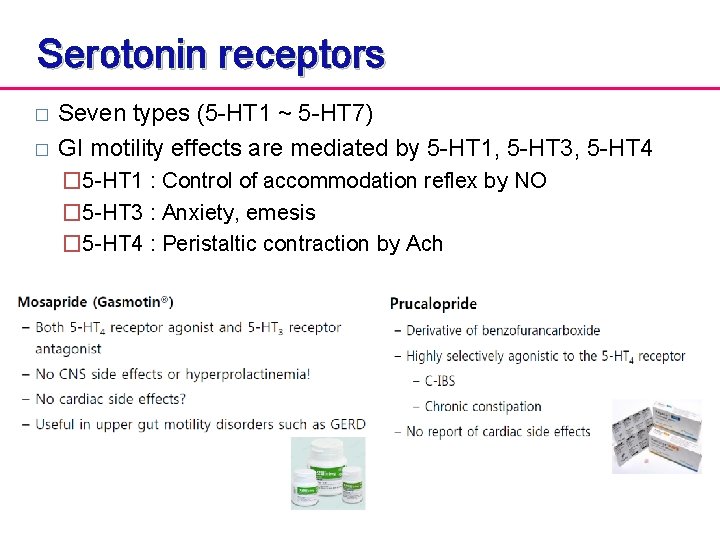
Serotonin receptors � � Seven types (5 -HT 1 ~ 5 -HT 7) GI motility effects are mediated by 5 -HT 1, 5 -HT 3, 5 -HT 4 � 5 -HT 1 : Control of accommodation reflex by NO � 5 -HT 3 : Anxiety, emesis � 5 -HT 4 : Peristaltic contraction by Ach


Effect of prokinetics is modest

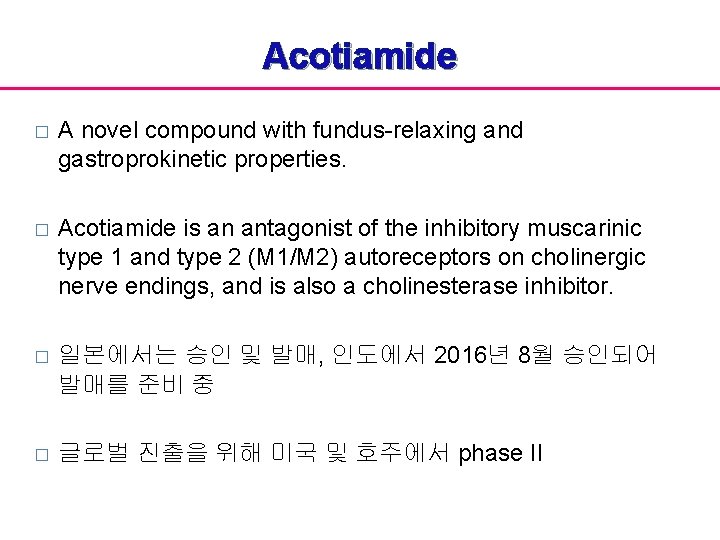
Acotiamide � A novel compound with fundus-relaxing and gastroprokinetic properties. � Acotiamide is an antagonist of the inhibitory muscarinic type 1 and type 2 (M 1/M 2) autoreceptors on cholinergic nerve endings, and is also a cholinesterase inhibitor. � 일본에서는 승인 및 발매, 인도에서 2016년 8월 승인되어 발매를 준비 중 � 글로벌 진출을 위해 미국 및 호주에서 phase II

Psychoactive medications SSRIs (ecitalopram) Improved Qo. L with this SSRI Serotonin 5 -HT 1 A receptor agonist (Buspirone) Improved the overall severity of FD symptoms (post-prandial fullness, early satiation, bloating) gastric accommodation↑ (relax the gastric fundus ) TCA (amitriptyline) Post-prandial nausea, adequate symptom relief in patients with ulcer-like FD Mirtazapine Over a placebo, FD with weight loss (appetite↑) Nausea/ vomiting Benzodiazepam (Clotiazepam) Depas (Etizolam) 항불안 효과

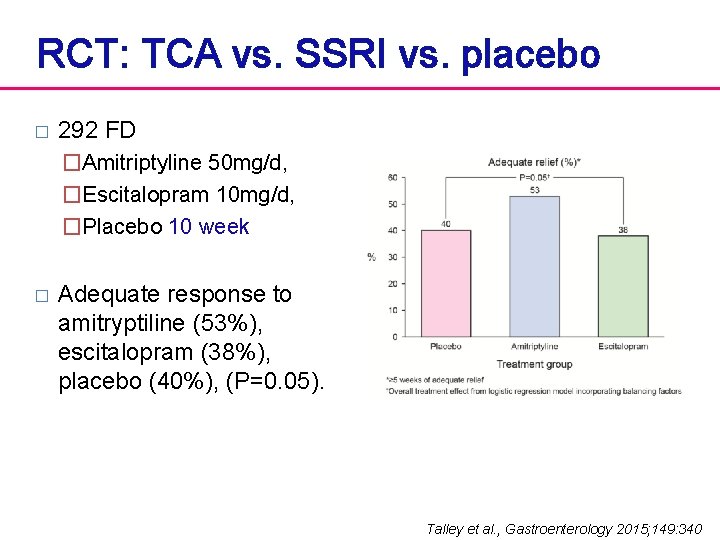
RCT: TCA vs. SSRI vs. placebo � 292 FD �Amitriptyline 50 mg/d, �Escitalopram 10 mg/d, �Placebo 10 week � Adequate response to amitryptiline (53%), escitalopram (38%), placebo (40%), (P=0. 05). Talley et al. , Gastroenterology 2015; 149: 340

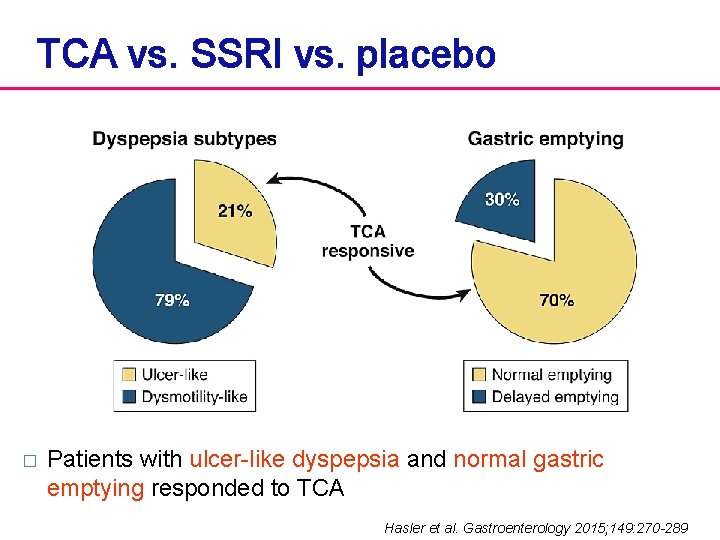
TCA vs. SSRI vs. placebo � Patients with ulcer-like dyspepsia and normal gastric emptying responded to TCA Hasler et al. Gastroenterology 2015; 149: 270 -289

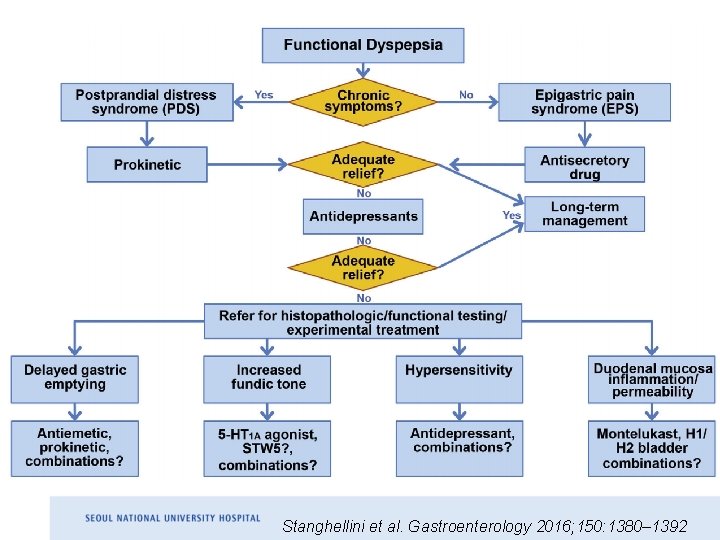
Stanghellini et al. Gastroenterology 2016; 150: 1380– 1392

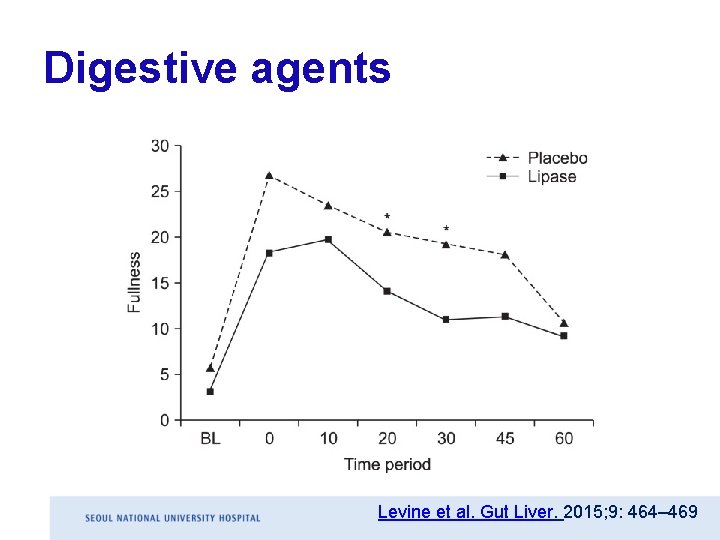
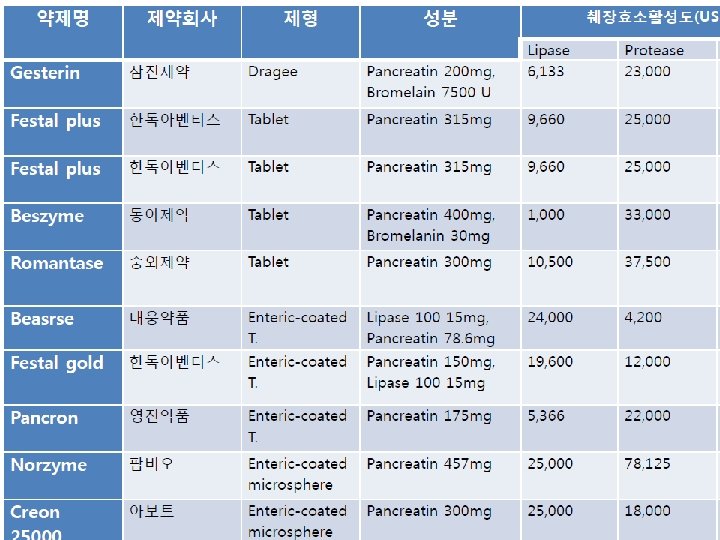
Digestive agents Levine et al. Gut Liver. 2015; 9: 464– 469


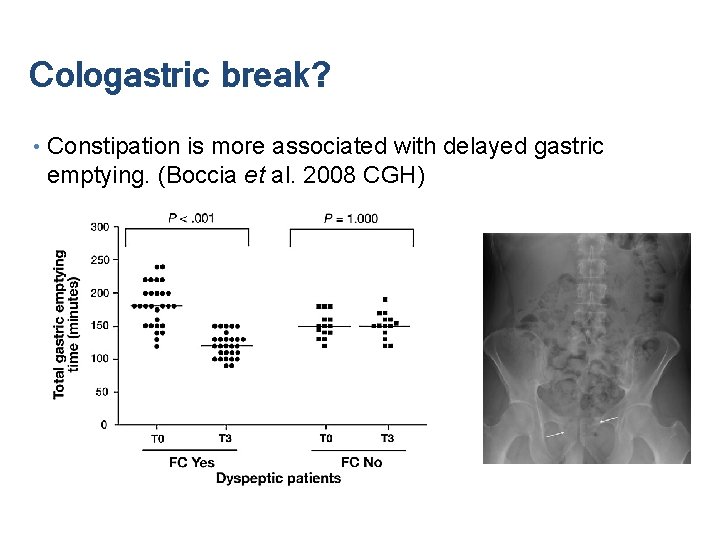
Cologastric break? • Constipation is more associated with delayed gastric emptying. (Boccia et al. 2008 CGH)

