Diagnostic and Research Utility of Whole Exome Sequencing
- Slides: 1

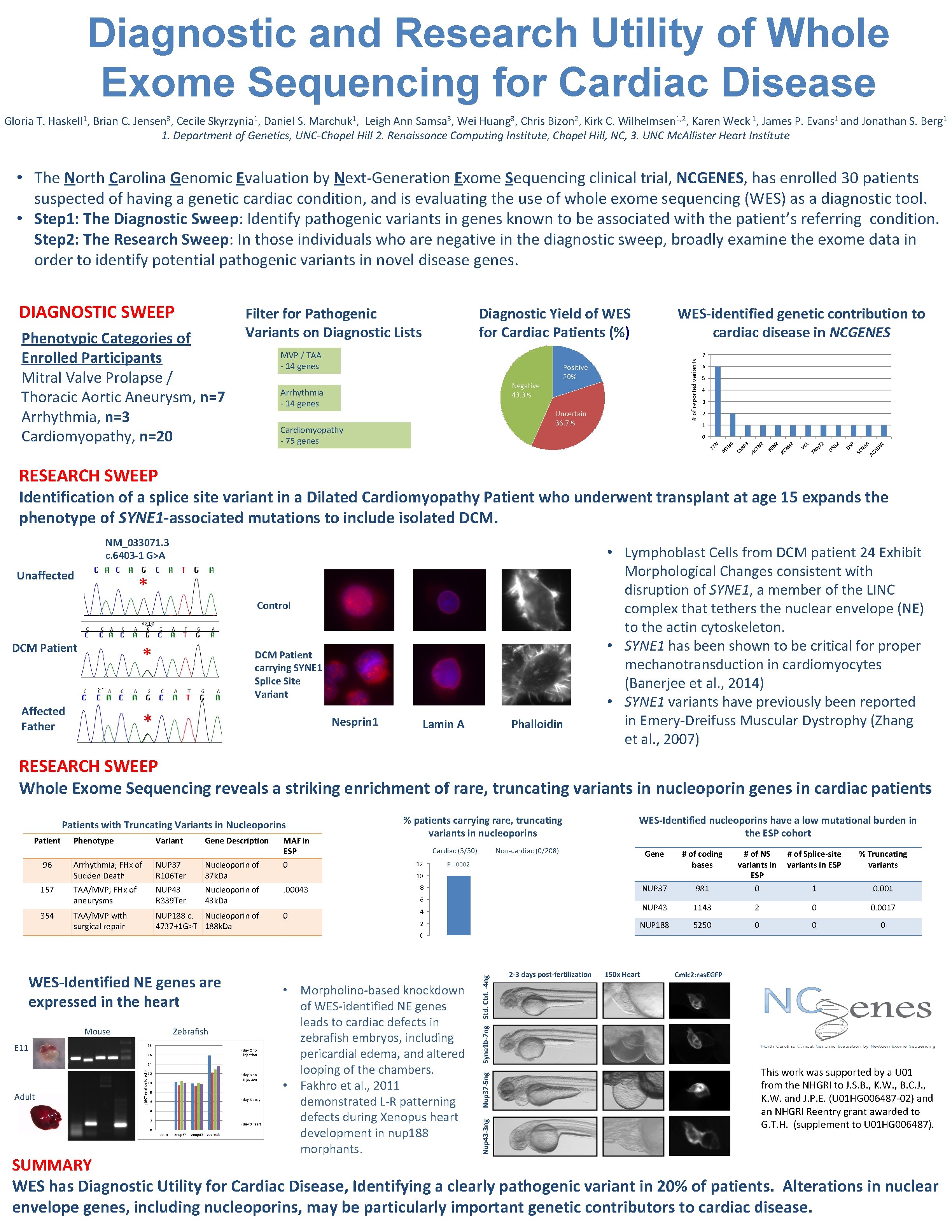
Diagnostic and Research Utility of Whole Exome Sequencing for Cardiac Disease Gloria T. Haskell 1, Brian C. Jensen 3, Cecile Skyrzynia 1, Daniel S. Marchuk 1, Leigh Ann Samsa 3, Wei Huang 3, Chris Bizon 2, Kirk C. Wilhelmsen 1, 2, Karen Weck 1, James P. Evans 1 and Jonathan S. Berg 1 1. Department of Genetics, UNC-Chapel Hill 2. Renaissance Computing Institute, Chapel Hill, NC, 3. UNC Mc. Allister Heart Institute • The North Carolina Genomic Evaluation by Next-Generation Exome Sequencing clinical trial, NCGENES, has enrolled 30 patients suspected of having a genetic cardiac condition, and is evaluating the use of whole exome sequencing (WES) as a diagnostic tool. • Step 1: The Diagnostic Sweep: Identify pathogenic variants in genes known to be associated with the patient’s referring condition. Step 2: The Research Sweep: In those individuals who are negative in the diagnostic sweep, broadly examine the exome data in order to identify potential pathogenic variants in novel disease genes. Diagnostic Yield of WES for Cardiac Patients (%) MVP / TAA - 14 genes WES-identified genetic contribution to cardiac disease in NCGENES 7 Positive 20% Negative 43. 3% Arrhythmia - 14 genes Uncertain 36. 7% Cardiomyopathy - 75 genes 6 5 4 3 2 1 AD VL A AC SC N 5 P DS 2 DS G NT 2 L TN VC NH 2 KC N 2 FB TN 2 AC RP 3 CS 6 M YH N 0 TT Phenotypic Categories of Enrolled Participants Mitral Valve Prolapse / Thoracic Aortic Aneurysm, n=7 Arrhythmia, n=3 Cardiomyopathy, n=20 Filter for Pathogenic Variants on Diagnostic Lists # of reported variants DIAGNOSTIC SWEEP RESEARCH SWEEP Identification of a splice site variant in a Dilated Cardiomyopathy Patient who underwent transplant at age 15 expands the phenotype of SYNE 1 -associated mutations to include isolated DCM. NM_033071. 3 c. 6403 -1 G>A Unaffected * Control DCM Patient * Affected Father * DCM Patient carrying SYNE 1 Splice Site Variant Nesprin 1 Lamin A Phalloidin • Lymphoblast Cells from DCM patient 24 Exhibit Morphological Changes consistent with disruption of SYNE 1, a member of the LINC complex that tethers the nuclear envelope (NE) to the actin cytoskeleton. • SYNE 1 has been shown to be critical for proper mechanotransduction in cardiomyocytes (Banerjee et al. , 2014) • SYNE 1 variants have previously been reported in Emery-Dreifuss Muscular Dystrophy (Zhang et al. , 2007) RESEARCH SWEEP Whole Exome Sequencing reveals a striking enrichment of rare, truncating variants in nucleoporin genes in cardiac patients Patients with Truncating Variants in Nucleoporins 354 Gene Description Arrhythmia; FHx of Sudden Death NUP 37 R 106 Ter Nucleoporin of 37 k. Da 0 TAA/MVP; FHx of aneurysms NUP 43 R 339 Ter Nucleoporin of 43 k. Da . 00043 TAA/MVP with surgical repair NUP 188 c. Nucleoporin of 4737+1 G>T 188 k. Da WES-Identified NE genes are expressed in the heart Mouse Zebrafish E 11 18 day 2 no injection 16 (-)d. CT relative to actin 14 Adult MAF in ESP 12 day 3 no injection 10 8 day 3 body 6 4 2 day 3 heart 0 actin znup 37 znup 43 zsyne 1 b Cardiac (3/30) WES-Identified nucleoporins have a low mutational burden in the ESP cohort Non-cardiac (0/208) Gene # of coding bases NUP 37 4 2 12 # of Splice-site variants in ESP % Truncating variants 981 # of NS variants in ESP 0 1 0. 001 NUP 43 1143 2 0 0. 0017 NUP 188 5250 0 P=. 0002 10 8 6 0 0 • Morpholino-based knockdown of WES-identified NE genes leads to cardiac defects in zebrafish embryos, including pericardial edema, and altered looping of the chambers. • Fakhro et al. , 2011 demonstrated L-R patterning defects during Xenopus heart development in nup 188 morphants. Syne 1 b-7 ng Std. Ctrl. -4 ng 157 Variant Nup 37 -5 ng 96 Phenotype Nup 43 -3 ng Patient % patients carrying rare, truncating variants in nucleoporins 2 -3 days post-fertilization 150 x Heart Cmlc 2: ras. EGFP This work was supported by a U 01 from the NHGRI to J. S. B. , K. W. , B. C. J. , K. W. and J. P. E. (U 01 HG 006487 -02) and an NHGRI Reentry grant awarded to G. T. H. (supplement to U 01 HG 006487). SUMMARY WES has Diagnostic Utility for Cardiac Disease, Identifying a clearly pathogenic variant in 20% of patients. Alterations in nuclear envelope genes, including nucleoporins, may be particularly important genetic contributors to cardiac disease.

