Diagnosis Precipitation Elements About H 2 O H

Diagnosis Precipitation Elements
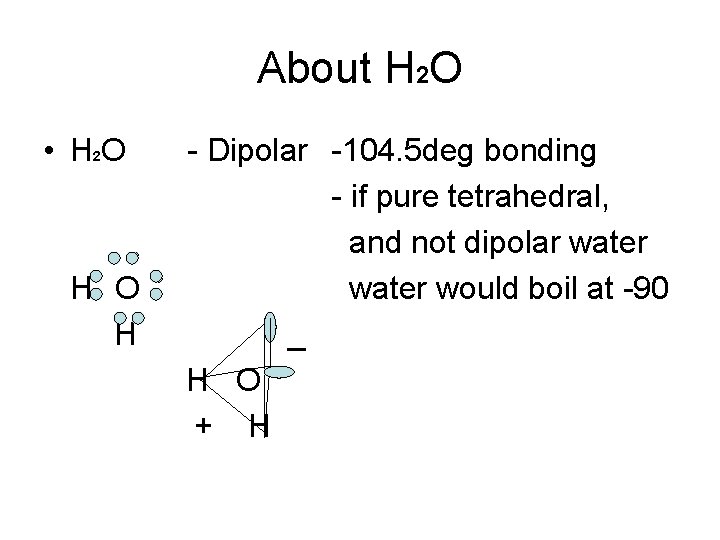
About H 2 O • H 2 O H - Dipolar -104. 5 deg bonding - if pure tetrahedral, and not dipolar water would boil at -90 _ H O + H
Formation of Cloud Droplets • -Clouds form when the atmosphere is saturated. • - (T = Td); the vapor pressure is equal to the saturation vapor pressure (e = es); and the relative humidity is 100 percent. • -Saturation is a state of equilibrium and water is constantly evaporating and condensing.
Formation of Cloud Droplets • Cloud condensation nuclei (CCN) are needed to facilitate the process of droplet formation through heterogeneous nucleation. ( *note) • CCNs include sea salt, fire smoke, volcanic sulfates clay and other dusts • The most effective nuclei are hygroscopic and may or man not be soluble • Cloud droplets are initiated by the condensation of on CCNs and continue to grow as water condenses onto the droplet.
Initial growth of droplets • The air immediately surrounding all the droplets is said to be saturated (e = es). • Initially, whether a droplet grows or not depends on saturation vapor pressure (es) of the droplet and its surrounding environment. • If es env > es droplet , the droplet grows due to condensation. If es env < es droplet, , the droplet shrinks through evaporation.
Curvature effect • Small droplets, which are tightly curved, have a larger saturation vapor pressure than larger droplets which are less curved (es small > es big) • If we have two droplets of different size the larger droplet grows at the expense of the smaller droplet.
Solute effect • In a cloud composed of pure water droplets of varying sizes, it is possible that water droplets below a certain critical size evaporate while larger droplets can grow by condensation. • But… in hetrogeneous nucleation cloud droplets form by condensation on "condensation nuclei"--some of which are hygroscopic. • The contamination of the droplet by the dissolved or solid hygroscopic substance changes the saturation vapor pressure. • Droplets consisting of higher concentrations of solution have a lower saturation vapor pressure than more pure droplets • As a result the solute effect and counteracts the curvature effect.

Collision and coalescence • Once droplets grow large enough to fall, they can collide with other droplets and merge to form even larger droplets. • Coalescence means the droplets which collide with each other stick together. However, drops can only become so big before they split apart into two or more drops. • These "new" drops, created by the splitting process, can then re-grow by collision-coalesence. • If this process is multiplied many, many times (as occurs in cumulonimbus clouds), the quantity of droplets increases dramatically. • Collision-coalescence is the most efficient method of droplet growth (much more efficient than condensation). Cloud to precip in 20 min with 100 droplets/cm 3 becoming 1000 drops/m 3 averaging 1 mm dia through 105 collisions. • Collision-coalescence is the dominant factor in warm air masses where cloud tops may not extend to temperatures < 0. (tropics)
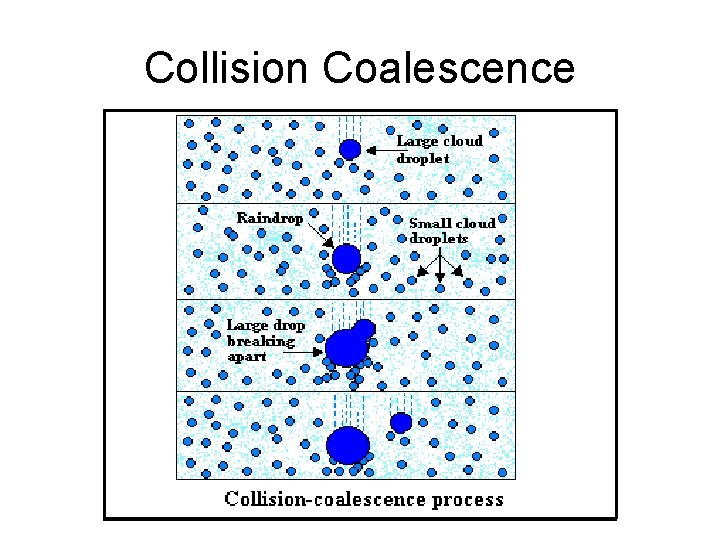
Collision Coalescence
Factors affecting collision and coalescence efficiency • Collision-coalescence is most efficient the longer a droplet remains in the cloud • Thick clouds, such as nimbostratus and cumulonimbus, provide a greater distance for the droplet to fall, thereby increasing coalescence time. • Updrafts associated with cumulus and cumulonimbus transport droplets to upper portions of the cloud and uncrease the distance and coalescence time of the droplet. Strong thunderstorm updrafts may exceed 20 ms-1, • Variable droplet fall velocities allow for more collisions. Larger droplets fall at a faster rate than smaller ones. A droplet with a diameter of 20 microns has a terminal velocity 0. 01 ms-1. A much larger droplet, with a diameter of 4, 000 microns, has a terminal velocity of 6. 5 ms-1.
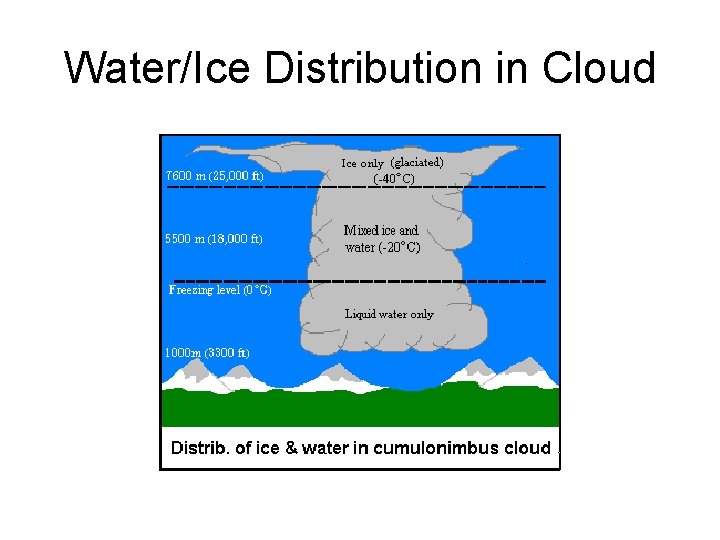
Water/Ice Distribution in Cloud

Formation and Growth of Ice Crystals • Once a cloud extends to altitudes where the temperature is <zero ice crystals may form. • Liquid droplets may freeze or direct deposition of vapour may occur on a solid phase ice crystal. (sublimation) • Ice crystals grow at the expense of liquid droplets. (Bergeron-Findeisen effect) es over ice is less than that over a water droplet, • Supersaturation with respect to ice is about 5% at -5 C and 20% at -20 C.
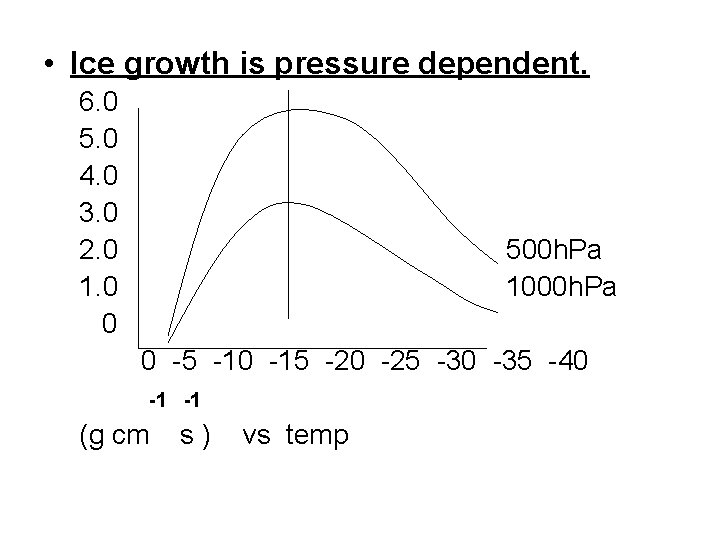
• Ice growth is pressure dependent. 6. 0 5. 0 4. 0 3. 0 2. 0 1. 0 0 500 h. Pa 1000 h. Pa 0 -5 -10 -15 -20 -25 -30 -35 -40 -1 -1 (g cm s) vs temp

Formation and Growth of Ice Crystals • Temp > -4 C = no ice crystals • Temp -12 to -14 = 75% have ice crystals • Temp < or = -20 C = certainty of ice

Other types of Ice growth • Accretion – falling ice crystals capture of super cooled water droplets. (lower levels) • Aggregation – ice crystals stick together. (most pronounced from -10 to Zero) • Rime Splintering – super cooled droplet develops frozen shell which expands and splinters producing more ice nuclei. Can glaciate a stratus cloud in 45 -90 minutes • Seeder-Feeder – Ice crystal cloud moves over a cloud of super cooled droplets. (Ice cloud < = 5000 ft above)
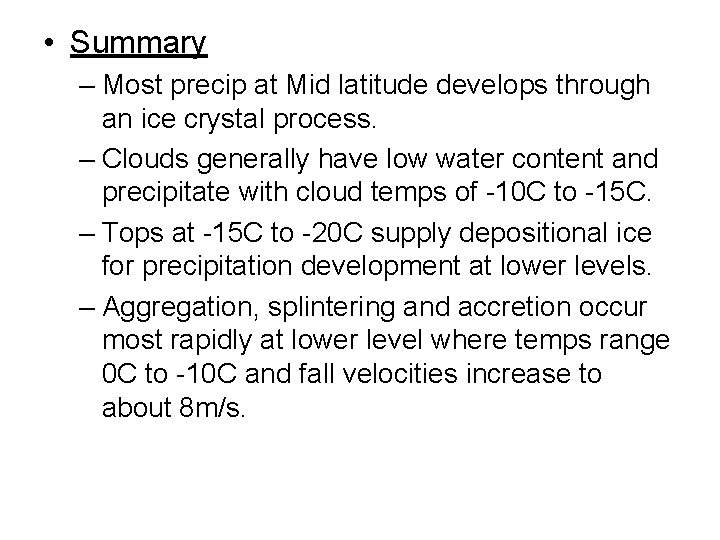
• Summary – Most precip at Mid latitude develops through an ice crystal process. – Clouds generally have low water content and precipitate with cloud temps of -10 C to -15 C. – Tops at -15 C to -20 C supply depositional ice for precipitation development at lower levels. – Aggregation, splintering and accretion occur most rapidly at lower level where temps range 0 C to -10 C and fall velocities increase to about 8 m/s.
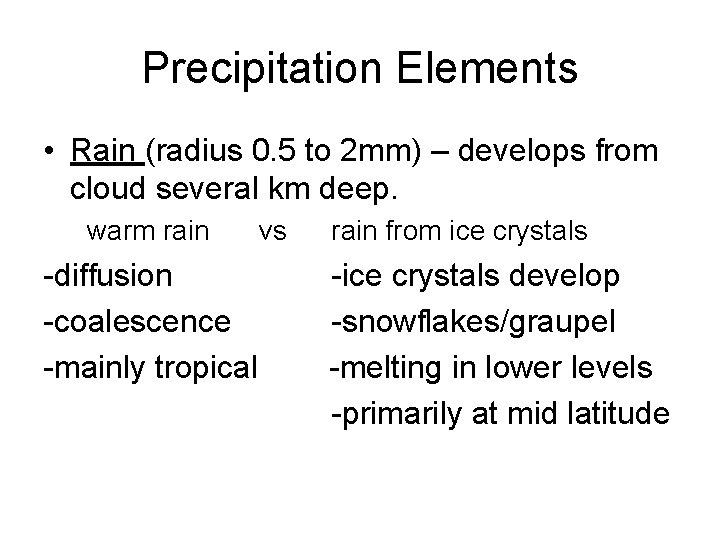
Precipitation Elements • Rain (radius 0. 5 to 2 mm) – develops from cloud several km deep. warm rain -diffusion -coalescence -mainly tropical vs rain from ice crystals -ice crystals develop -snowflakes/graupel -melting in lower levels -primarily at mid latitude

• Drizzle - 0. 1 mm – develops from cloud 1 km deep or less. (sloping shore line) • Snow – develop from ice crystal diffusion process then growth by aggregation (near zero) • Snow Pellets – ice crystal or frozen water droplet accretes super cooled water to form rimmed structure. (Graupel) Typically 2 -7 mm from spring TCU. • Hail – graupel accretes wet layer of super cooled water forming hard clear ice near 0 C due to latent heat release. Typically 5 to 30 mm with available liquid cloud water. Forming in CB.

• Ice crystals – develop at low temperature with growth by diffusion. Various shapes. • Snow Grains – solid equivalent of drizzle. Fall from relatively thin stratus. • Freezing Rain – Super cooled rain droplets that freeze upon contact. • Ice Pellets – Formation similar to freezing rain but freeze before ground contact. • Freezing Drizzle – Typically 0 C to -10 C droplets freezing on contact. No above freezing layer aloft necessary.

The Top Down Approach • Diagnose precipitation type/phase. - Cool mid level air – ice producing - Elevated warm layer – maybe melting - Cold surface layer – Refreeze/contact • See Check list. Page 11 Precipitation Element handout.
Cloud dynamics • Generally, cloud types and amounts are determined by – the amount of atmospheric moisture available – Temperature – Stability – Lifting mechanisms.

• First and foremost, there must be enough available atmospheric moisture present for clouds to form. No amount of lift or cooling will produce clouds if sufficient moisture is not present.
- Slides: 22