Diabetes mellitus A group of metabolic disorders sharing

Diabetes mellitus A group of metabolic disorders sharing the common feature of hyperglycemia Ghadeer hayel, MD
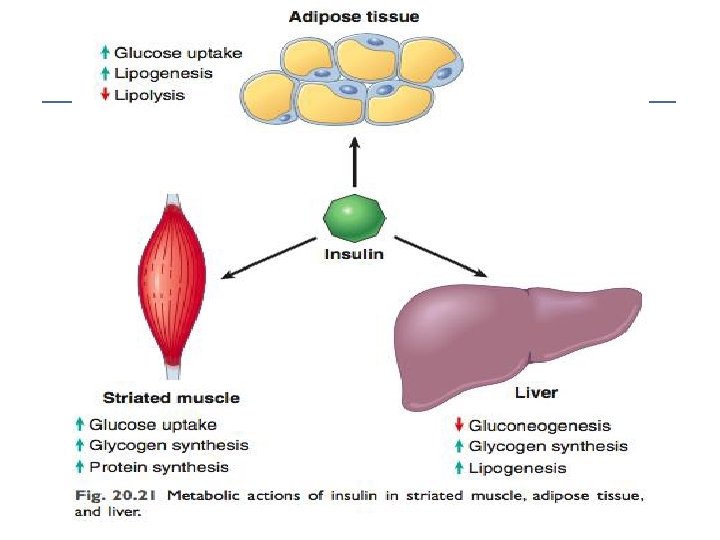
Insulin function The principal function of insulin is to increase the rate of glucose transport into certain cells in the body. Metabolic effects of insulin anabolic: Increased synthesis & reduced degradation of glycogen, lipid, & protein. Initiation of DNA synthesis in certain cells Stimulating their growth & differentiation.
Types of Diabetes Malitus The vast majority of cases of diabetes fall into one of two broad classes: q Type 1: an autoimmune disease pancreatic β-cell destruction absolute deficiency of insulin q Type 2: a combination of peripheral resistance to insulin & an inadequate secretory response by the pancreatic β cells (relative insulin deficiency).
Type 1 Diabetes 5% to 10% of DM cases The most common subtype diagnosed in children (<20 age), but can present any age. An autoimmune destruction to islets caused primarily by immune effector cells reacting against endogenous beta cell antigens. Patients depend on exogenous insulin for survival Classic manifestations of the disease occur late after > 90% of the beta cells have been destroyed.

Type 1 Diabetes Pathogenesis involves genetic susceptibility & environmental factors ( viral infections) Defective clonal deletion of self-reactive T cells in the thymus and abnormalities of regulatory T cells production of autoantibodies against a variety of beta cell antigens (insulin & the beta cell enzymes)
Type 2 Diabetes A heterogeneous & multifactorial complex disease involves interactions of genetics, environmental risk factors, & inflammation. ~ 90% of DM, usually adult onset ? MAIN two defects in type 2 DM: (1) a decreased ability of peripheral tissues to respond to insulin (insulin resistance). (2) beta cell dysfunction inadequate insulin secretion in the face of insulin resistance & hyperglycemia.
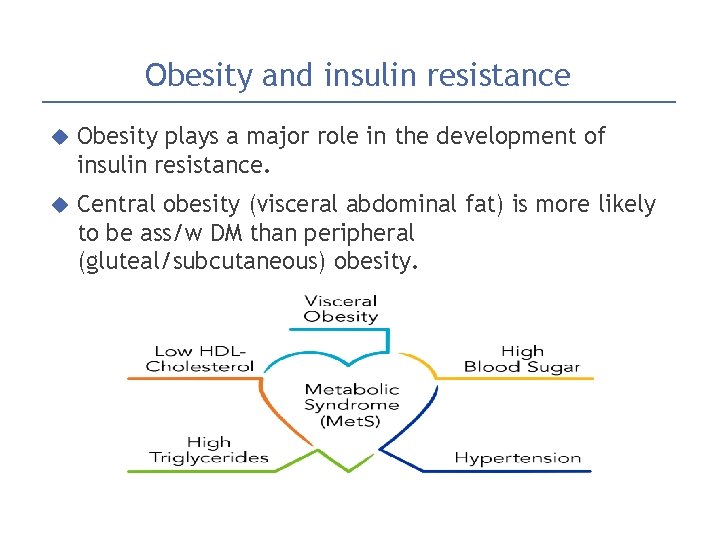
Obesity and insulin resistance Obesity plays a major role in the development of insulin resistance. Central obesity (visceral abdominal fat) is more likely to be ass/w DM than peripheral (gluteal/subcutaneous) obesity.

Obesity and insulin resistance 1. excess circulating FFAs (free fatty acids)�taken up into organs (muscle, liver)�↑↑ intracellular triglycerides � potent inhibitors of insulin signaling �acquired insulin resistance. 2. Adipokines (adipose cytokines): one important is adiponectin �decrease blood glucose by increasing the insulin sensitivity of peripheral tissues. Adiponectin levels are decreased in obesity 3. Proinflammatory cytokines that are secreted in response to excess nutrients such as FFAs results in both peripheral insulin resistance & beta cell dysfunction
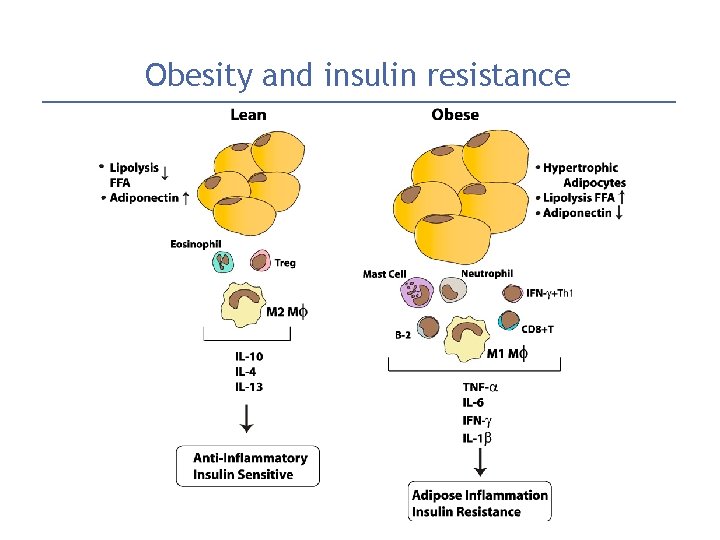
Obesity and insulin resistance
Beta Cell Dysfunction Beta cell dysfunction is an essential component in the development of overt diabetes. Beta cell function increases early in the disease process of type 2 diabetes �as a compensatory measure to counter insulin resistance & maintain euglycemic. But beta cells are unable to adapt to the long-term demands of peripheral insulin. Also excess free fatty acids compromise beta cell function & attenuate insulin release.
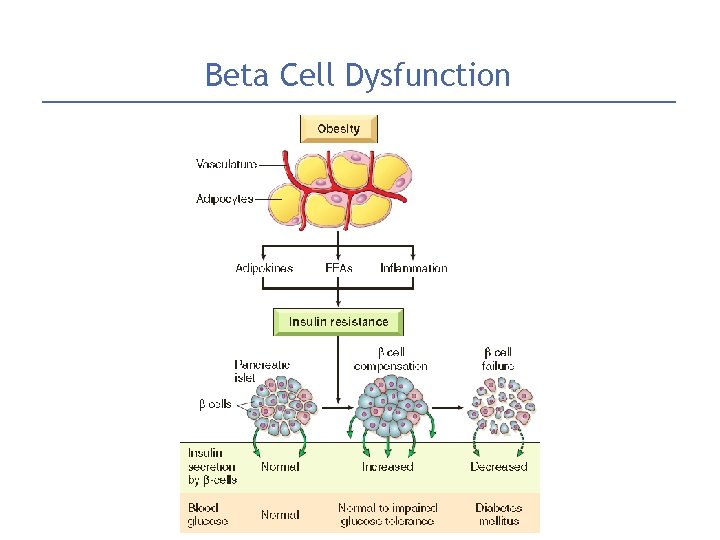
Beta Cell Dysfunction
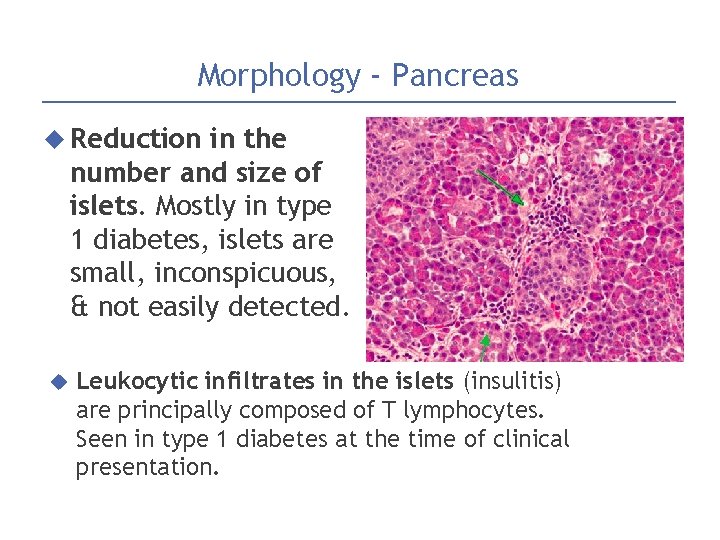
Morphology - Pancreas Reduction in the number and size of islets. Mostly in type 1 diabetes, islets are small, inconspicuous, & not easily detected. Leukocytic infiltrates in the islets (insulitis) are principally composed of T lymphocytes. Seen in type 1 diabetes at the time of clinical presentation.
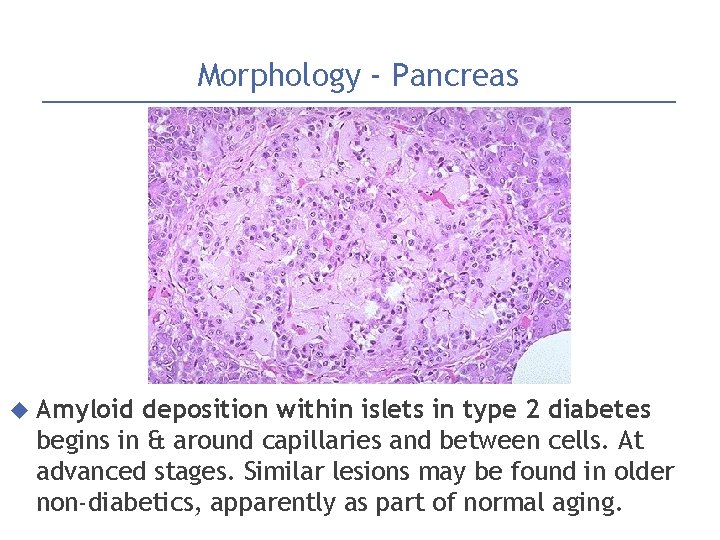
Morphology - Pancreas Amyloid deposition within islets in type 2 diabetes begins in & around capillaries and between cells. At advanced stages. Similar lesions may be found in older non-diabetics, apparently as part of normal aging.
Clinical: Initial Presentation The classic triad of diabetes a. The hyperglycemia exceeds the renal threshold for reabsorption, & glycosuria induces an osmotic diuresis polyuria b. The renal water loss combined with the hyperosmolarity (↑↑↑ blood glucose) deplete intracellular water trigger thirst centers in brain intense thirst polydipsia c. Deficiency of insulin leads to catabolism of proteins & fats induce a negative energy balance increasing appetite polyphagia

Clinical: Diabetic ketoacidosis A severe acute metabolic complication of type 1 diabetes (rare in type 2) Major effect of insulin deficiency is activation of ketogenic machinery: Insulin def. �stimulate lipoprotein lipase �breakdown of adipose stores�eventually ↑↑↑ levels ketone bodies. The rate at which ketone bodies are formed exceed the rate of utilization by peripheral tissues + urinary excretion of ketones is compromised by dehydration. The result is a systemic metabolic ketoacidosis.

Chronic Complications of Diabetes Diabetic macrovascular disease: 1. Accelerated atherosclerosis, more severe & earlier onset in DM. 2. Myocardial infarction, caused by atherosclerosis of the coronary arteries, is the most common cause of death in diabetics. 3. Gangrene of the lower extremities: 100 times more common in diabetics than in the general population.
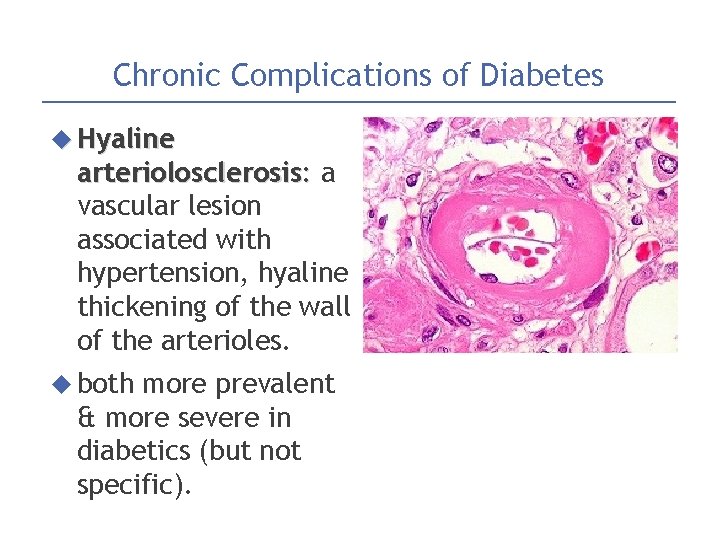
Chronic Complications of Diabetes Hyaline arteriolosclerosis: a vascular lesion associated with hypertension, hyaline thickening of the wall of the arterioles. both more prevalent & more severe in diabetics (but not specific).
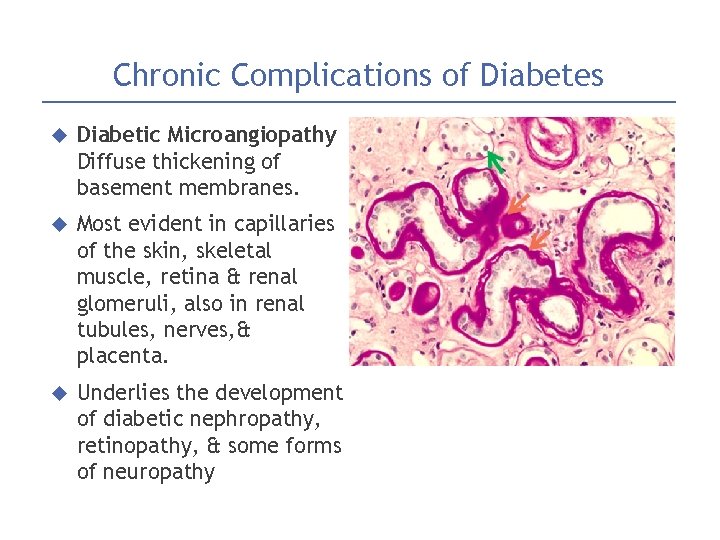
Chronic Complications of Diabetes Diabetic Microangiopathy Diffuse thickening of basement membranes. Most evident in capillaries of the skin, skeletal muscle, retina & renal glomeruli, also in renal tubules, nerves, & placenta. Underlies the development of diabetic nephropathy, retinopathy, & some forms of neuropathy

Chronic Complications of Diabetes Diabetic Nephropathy The kidneys are prime targets of DM. Renal failure is second only to MI as a cause of death in DM. Three lesions are encountered: 1) Glomerular 2) Renal lesions vascular lesions, arteriolosclerosis 3) Pyelonephritis

Chronic Complications of Diabetes Diabetic neuropathy. The central & peripheral nervous systems are not spared by diabetes. The most frequent pattern is symmetric neuropathy of the lower extremities affecting both motor & sensory function. (tingling, burning or prickling sensation in feet & hands) Result from microangiopathy & direct axonal damage

Chronic Complications of Diabetes Diabetic Retinopathy Visual impairment & blindness, one of the more feared consequences of long-standing DM. (fourth leading cause of acquired blindness in US) 60% - 80% of patients develop a form of diabetic retinopathy in 15 to 20 of diagnosis Diabetic patients also have an increased propensity for glaucoma & cataract formation
- Slides: 23