Diabetes in Pregnancy Diagnosis and Management MARIA PALMQUIST

Diabetes in Pregnancy: Diagnosis and Management MARIA PALMQUIST, MD SEPTEMBER 22, 2017

Objectives To review current trends in the diagnosis of gestational diabetes ◦ Risk factors for development of gestational diabetes ◦ Timing of screening To review benefits of treating gestational diabetes To review the ideal components of a diabetic diet ◦ Caloric targets ◦ Carbohydrate portions To review the use of insulin and oral agents in gestational diabetes

Pathophysiology of Diabetes in Pregnancy = Insulin Resistance Mediated by placental hormones ◦ ◦ Growth hormone CRH Placental lactogen Progesterone GDM develops in those women whose pancreatic function insufficient to overcome the insulin resistance associated with pregnancy Shortage of insulin/hyperglycemia ◦ Preeclampsia, macrosomia, cesarean section, and associated morbidities
Diabetes in Pregnancy Historically GDM defined as onset or first recognition of abnormal glucose tolerance during pregnancy ◦ ACOG continues to use this terminology ◦ IADPSG, ADA, WHO and others attempt to distinguish women with probable preexisting diabetes ◦ Acknowledging the increased prevalence of undiagnosed type 2 diabetes ◦ Gestational diabetes = diagnosed second half of pregnancy ◦ Overt diabetes = diabetes diagnosed in early pregnancy, using the standard non-pregnant criteria Prevalence of GDM historically 6 -7% in the US (range 1 -25%) ◦ Using IADPSG screening ~17%

Prevalence of Diabetes Around the World Prevalence of GDM in direct proportion to the prevalence of type 2 diabetes Prevalence varies worldwide and among racial and ethnic groups Prevalence varies because of: ◦ ◦ Screening practices Populations characteristics, obesity Testing methods Diagnostic criteria Prevalence increasing over time: ◦ Mean maternal age increasing ◦ Mean maternal weight increasing

Rates of Diagnosed Diabetes by Race • 7. 6 % of non-Hispanic whites • 9. 0% of Asian Americans • 12. 8% of Hispanics • 13. 2% of non-Hispanic blacks • 15. 9% of American Indians/Alaskan Natives
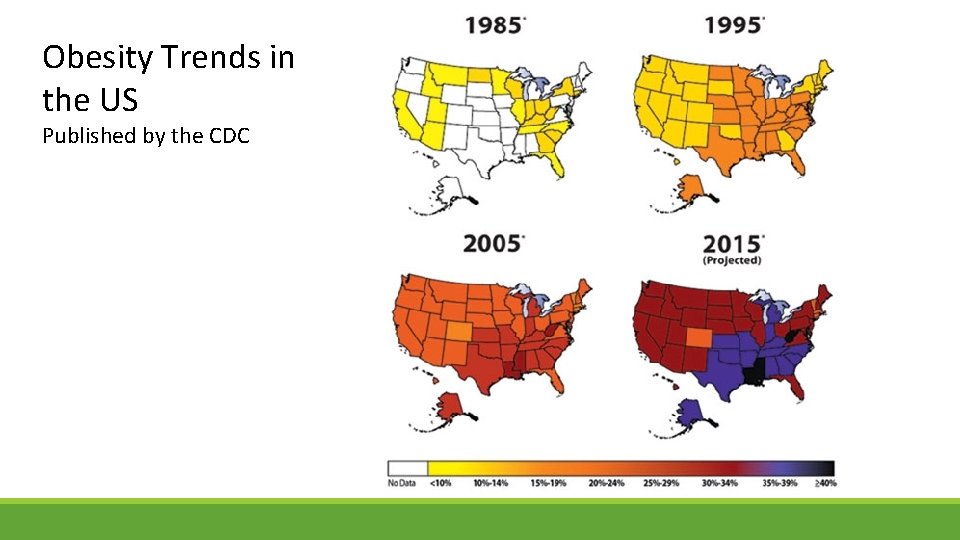
Obesity Trends in the US Published by the CDC
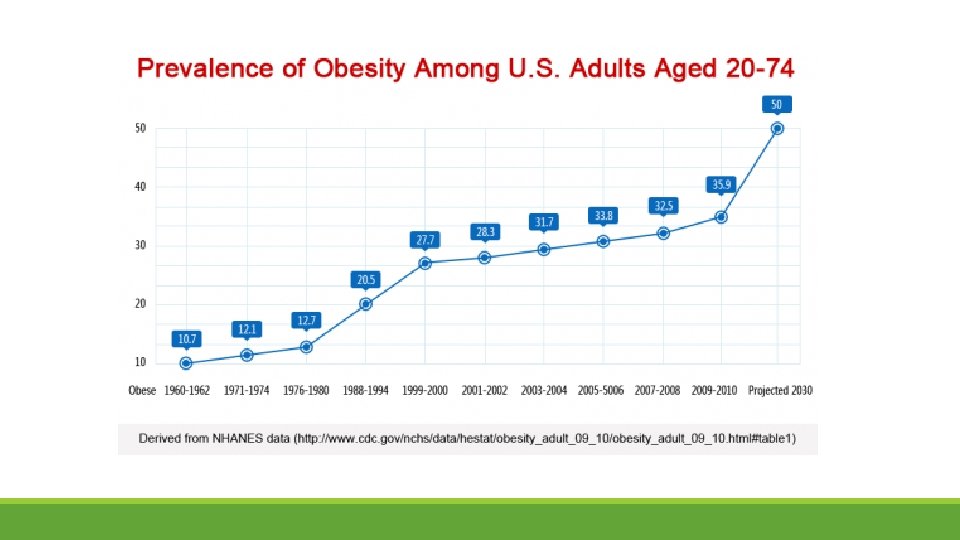
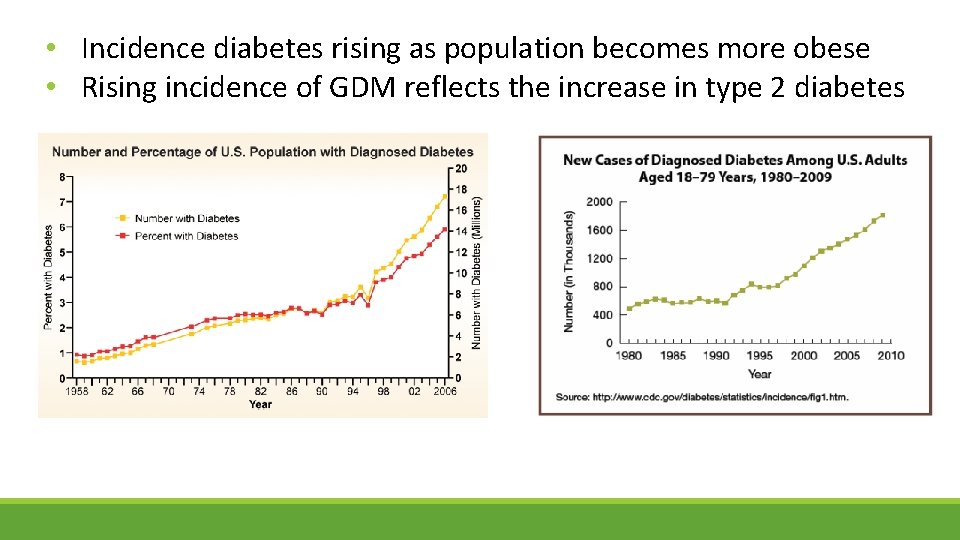
• Incidence diabetes rising as population becomes more obese • Rising incidence of GDM reflects the increase in type 2 diabetes

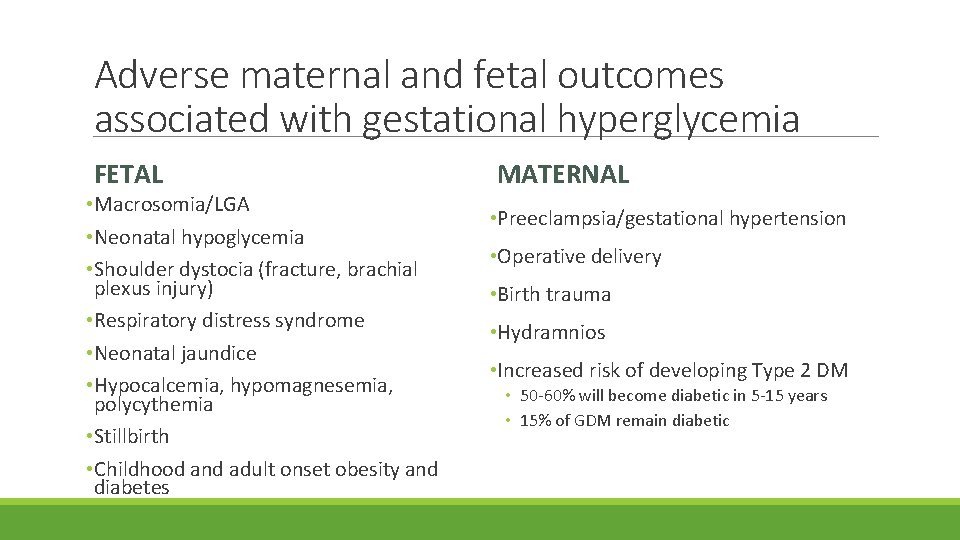
Adverse maternal and fetal outcomes associated with gestational hyperglycemia FETAL • Macrosomia/LGA • Neonatal hypoglycemia • Shoulder dystocia (fracture, brachial plexus injury) • Respiratory distress syndrome • Neonatal jaundice • Hypocalcemia, hypomagnesemia, polycythemia • Stillbirth • Childhood and adult onset obesity and diabetes MATERNAL • Preeclampsia/gestational hypertension • Operative delivery • Birth trauma • Hydramnios • Increased risk of developing Type 2 DM • 50 -60% will become diabetic in 5 -15 years • 15% of GDM remain diabetic

Gestational Diabetes Screening/Testing • Treatment of GDM can reduce the risk of complications: preeclampsia and adverse neonatal outcomes • Risk factors: personal history of GDM, high risk ethnic group, obese, family history, previous macrosomic infant, medical conditions (PCOS, steroid use, hypertension, elevated cholesterol/triglycerides), multiple gestation • Only 10% of general obstetric population in US low risk • Approaches for risk reduction: prepregnancy weight loss • Cost models show screening cost-effective for prevention of type 2 diabetes, provided lifestyle interventions are applied subsequent to pregnancy, and in improving maternal and neonatal outcomes.
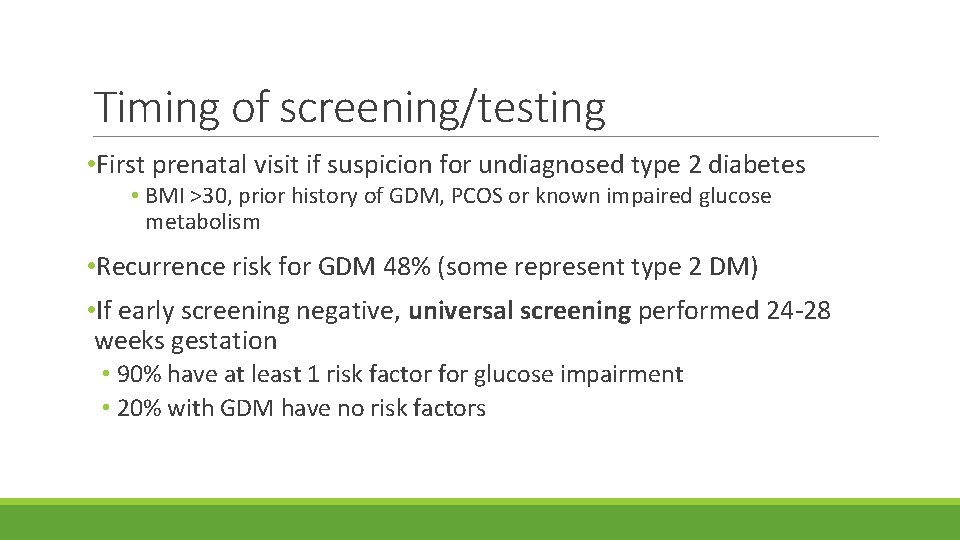
Timing of screening/testing • First prenatal visit if suspicion for undiagnosed type 2 diabetes • BMI >30, prior history of GDM, PCOS or known impaired glucose metabolism • Recurrence risk for GDM 48% (some represent type 2 DM) • If early screening negative, universal screening performed 24 -28 weeks gestation • 90% have at least 1 risk factor for glucose impairment • 20% with GDM have no risk factors
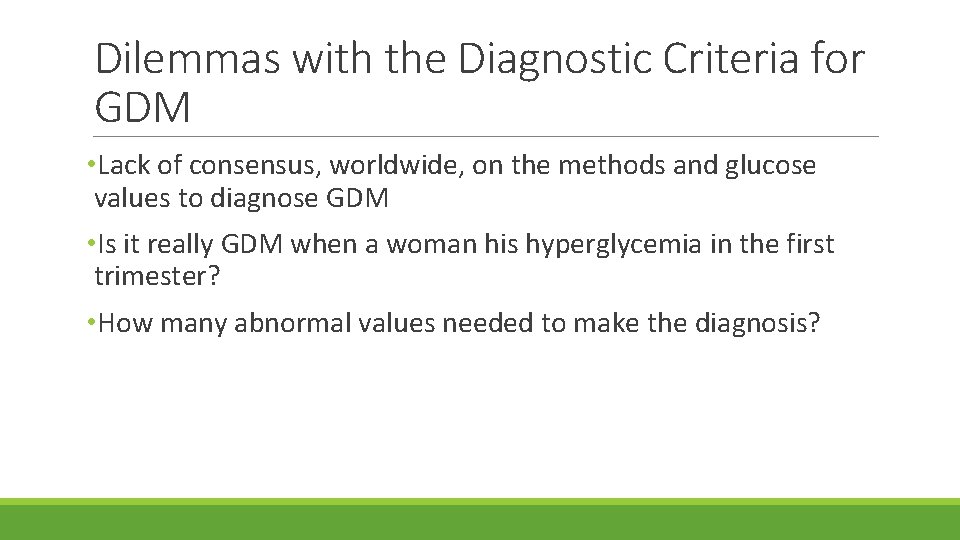
Dilemmas with the Diagnostic Criteria for GDM • Lack of consensus, worldwide, on the methods and glucose values to diagnose GDM • Is it really GDM when a woman his hyperglycemia in the first trimester? • How many abnormal values needed to make the diagnosis?

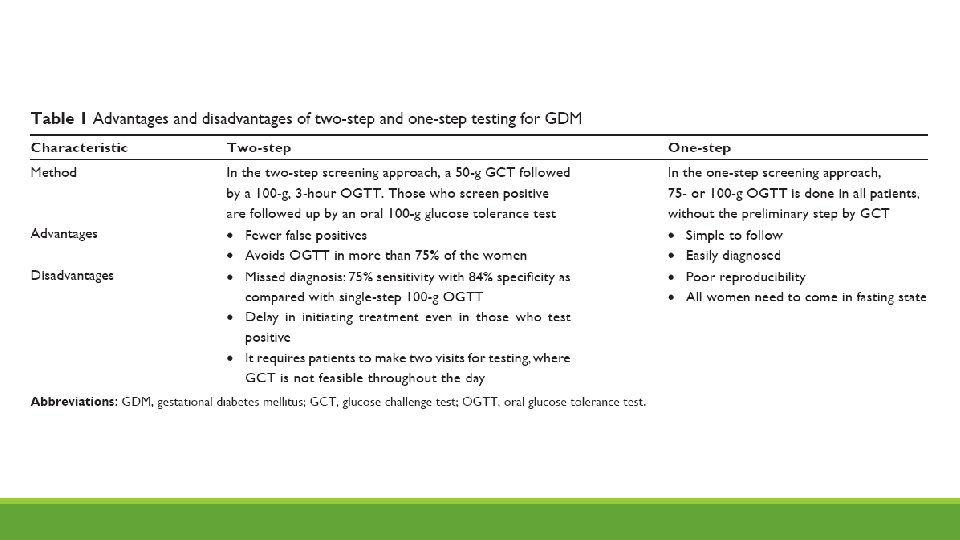
2 Step Testing: most widely used in US • First step identifies those at increased risk for GDM. • Second step (diagnostic test) limited to those at increased risk • 24 -28 weeks gestation Step 1. ◦ 1 hour 50 gram glucose challenge ◦ No fasting necessary ◦ >140 identifies 80% of women with GDM, >130 identifies 90% women with GDM Step 2. ◦ 3 hour 100 gram GTT ◦ Overnight fast ◦ Women with 1 abnormal value at increased risk for poor outcomes
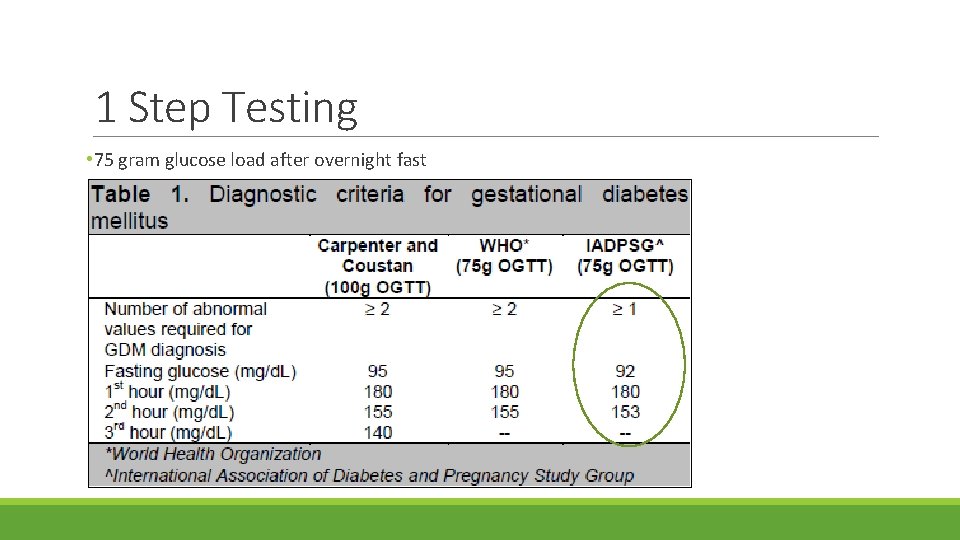
1 Step Testing • 75 gram glucose load after overnight fast
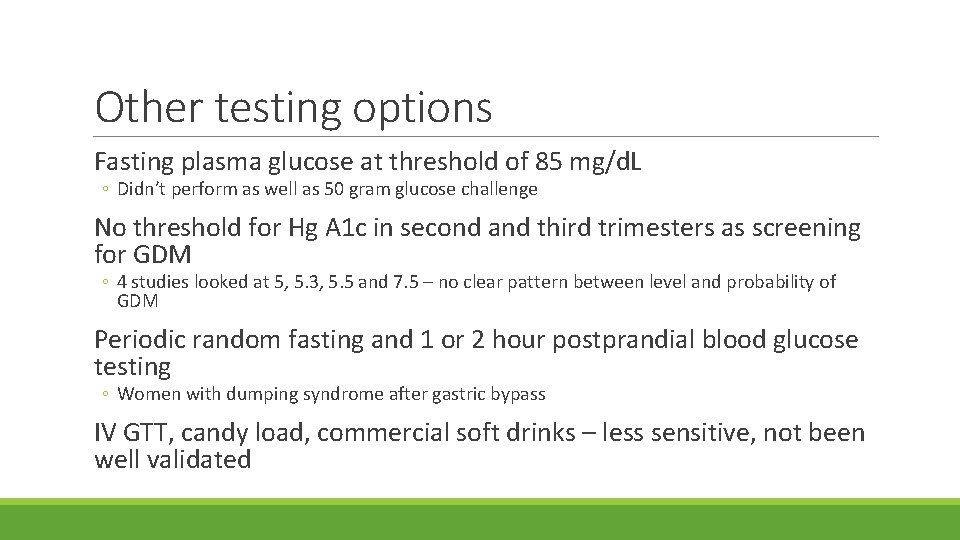
Other testing options Fasting plasma glucose at threshold of 85 mg/d. L ◦ Didn’t perform as well as 50 gram glucose challenge No threshold for Hg A 1 c in second and third trimesters as screening for GDM ◦ 4 studies looked at 5, 5. 3, 5. 5 and 7. 5 – no clear pattern between level and probability of GDM Periodic random fasting and 1 or 2 hour postprandial blood glucose testing ◦ Women with dumping syndrome after gastric bypass IV GTT, candy load, commercial soft drinks – less sensitive, not been well validated

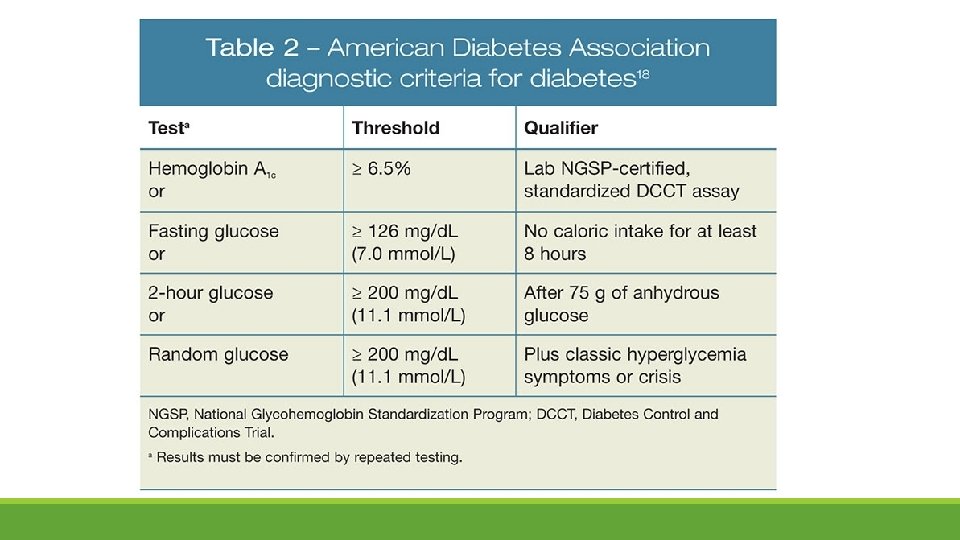
Overt Diabetes Early in Pregnancy • Increasing number of women have unrecognized type 2 diabetes due to increasing obesity and lack of routine glucose screening/testing in this age group • Increased risk for fetal congenital anomalies and maternal nephropathy or retinopathy • ACOG supports screening in women with risk factors: previous GDM, obesity, PCOS • Test for undiagnosed preexisting diabetes at the first prenatal visit • WHO, IADPSG, ADA…. allow for diagnosis of overt diabetes ◦ If meet criteria for diabetes early in pregnancy, before significant insulin resistance develops

Which method is best?

American Congress of Obstetricians and Gynecologists (ACOG) • Supports 2 step approach • Step one • Give 50 gram oral glucose load any time of day • Thresholds for 1 hour test vary from >130 to >140; if elevated requires 100 gram GTT • Step two • Measure fasting glucose • Give 100 gram glucose load • Measure serum glucose 1 hour, 2 hour and 3 hours after glucose load • Generally a positive test is defined as 2 elevated values, but one abnormal value may be used for the diagnosis of GDM • It is estimated that if 75 g single step testing is implemented across the US, the prevalence of GDM is expected to increase to >20 percent.

One-step approach • In 2010, International Association of Diabetes and Pregnancy study group (IADPSG) recommended universal 75 g, 2 hour GTT • Would identify 18% of pregnant women in the US as having GDM • Adequate resources may present a challenge • In 2011, American Diabetes Association (ADA) endorsed the one-step approach ◦ 2017, ADA recognized absence of evidence to support one approach over the other ◦ ADA supports both approaches • World Health organization (WHO), The Endocrine Society, Australian Diabetes in Pregnancy Society support one-step approach • A 2015 Cochrane review supported that no specific screening strategy has been shown to be optimal.
Rationale for Treatment • Prevent fetal death • Prevent LGA and macrosomia • Reduce rate of shoulder dystocia and birth trauma • Brachial plexus injury, fracture, neonatal depression • Reduce number of operative deliveries • Reduce rate of maternal preeclampsia • Reduce neonatal morbidities • Hypoglycemia, hyperbilirubinemia, respiratory distress • Hypocalcemia, hypomagnesemia, polycythemia • Prevent development of type 2 DM

Medical Nutrition Therapy Goals §Provide necessary nutrition for fetal and maternal well-being §Achieve normoglycemia § Fasting 60 -90 mg/dl § 1 hr after meals <140 mg/dl § 2 hr after meals <120 mg/dl §Allow for adequate weight gain §Prevent ketosis
Dietary Recommendations • 3 meals and 3 snacks • Limit CHO intake to 30 -40%, protein 20%, fat 40% • CHO restriction at breakfast • Caloric distribution: 10% breakfast, 20 -30% for lunch 30 -40% dinner, 30% snacks • Eliminate liquid CHO (juices) • Avoid high glycemic index foods • Increase high fiber foods (25 -30 grams) • Exercise • Limit weight gain
Carbohydrate Budget §Breakfast 30 grams (2 choices) §Lunch 45 -60 grams (3 -4 choices) §Dinner 45 -60 grams (3 -4 choices) §Snacks (1 -3) 15 -30 grams (1 -2 choices) §Amount of CHO typically found in 2200 calorie diet
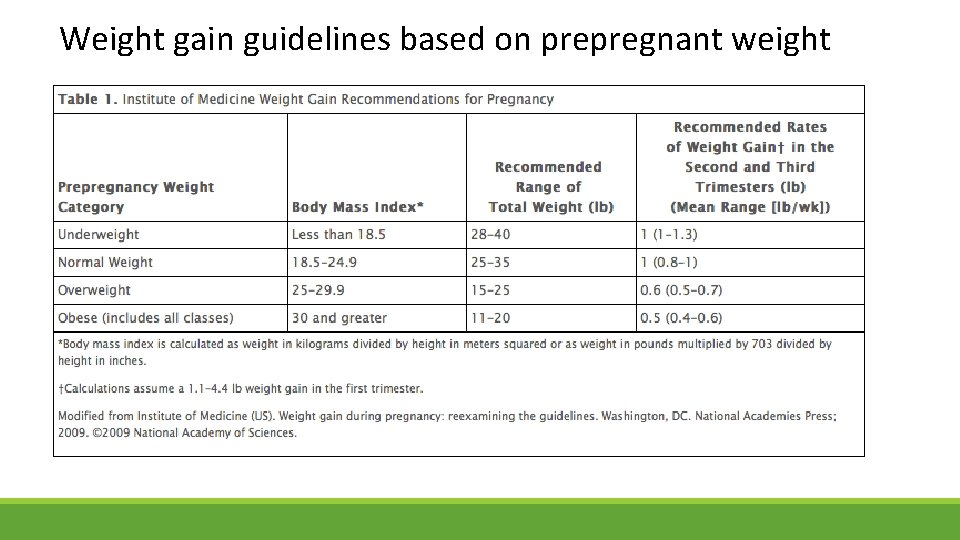
Weight gain guidelines based on prepregnant weight
Glucose Monitoring • Monitor blood sugars four times daily: fasting and after each meal • No controlled trials to identify optimal glycemic targets • ADA and ACOG support: • FBS < 95 • 1 hour PP < 140 • 2 hour PP < 120 • The optimal time for postprandial monitoring unclear • Consider decreasing testing frequency when good glycemic control • Every other day • Two measurements/day

When to initiate pharmocotherapy Target glucoses cannot be consistently achieved All dietary modifications have been implemented ◦ Allow approximately 2 weeks Sonogram findings to consider ◦ LGA ◦ Fetal AC > 75%ile at 29 -33 weeks (Buchanan diabetes care 1998) ◦ Polyhydramnios No conclusive evidence for threshold values to start ◦ >50% values elevated certain time of day ◦ 15 -20% all values abnormal

Insulin therapy • Historically considered standard of care • Does not cross the placenta • More physiologic • Allows for fine tuning/tight control • Typical starting doses 0. 7 -1. 0 units/kg daily, given in divided doses • Mild hyperglycemia ◦ FBS elevated - start NPH 0. 2 units per kg at bedtime ◦ 140 -150 s PP – start Humalog 4 -6 units before meals • Individualize according to the patient
Metformin • Insulin sensitizer • Maximum dose usually 2, 500 -3, 000 mg/day. Starting dose 500 mg nightly. • Crosses the placenta, levels as high as maternal concentrations • Doesn’t cause hypoglycemia • Most common side effect is GI upset • Typically used in women with pregestational diabetes and/or PCOS • Transition type 2 patients to insulin when pregnancy confirmed • In PCOS, discontinue after first trimester and start insulin • Between 26 -46% eventually require insulin to achieve glycemic control • Randomized trial - found increased risk for preterm birth (risk ratio 1. 5) in metformin group, but less gestational weight gain • Lack of long-term data in exposed offspring
Glyburide • Binds to the pancreatic receptors to increase insulin secretion and increases insulin sensitivity of peripheral tissues • It’s a sulfonylurea, avoid in patients with sulfa allergy • Compared to insulin: higher rates of respiratory distress, hypoglycemia, macrosomia and birth injury • Common dosage is 2. 5 -20 mg daily in divided doses • Pharmacokinetics indicated 30 mg daily may be necessary to achieve adequate control • Long tail – most common side effect is hypoglycemia • 10 -20% require the addition of insulin to maintain good glycemic control • Should not be offered as first-line treatment because it does not yield equivalent outcomes to insulin

Medication Management Pearls §There must always be a combination of medicine and diet §Starting medication is just that. A start. Patient will not be controlled immediately. It will have to be adjusted. Especially with insulin. §Exercise makes everything better §Oral agents won’t work if blood glucose consistently > 170 mg/dl §Beware of vicious cycle. Increase blood glucose -> increase insulin increase weight gain -> increase insulin resistance

Antenatal Care • NSTs or weekly BPP starting at 32 weeks gestation for A 2 GDM • Serial growth scans beginning at 28 weeks • 28, 32, 38 weeks ideal – to assist with delivery plans • Induction of labor at 39 weeks with A 2 GDM • No benefit in going beyond 40 weeks • Risk for shoulder dystocia increases 10% with expectant management • Counsel on risks/benefits of c-section with an EFW >4, 500 grams • Goal for blood glucoses at 70 -120 mg/dl during labor • Most important determinant of neonatal hypoglycemia • >140 associated with neonatal hypoglycemia
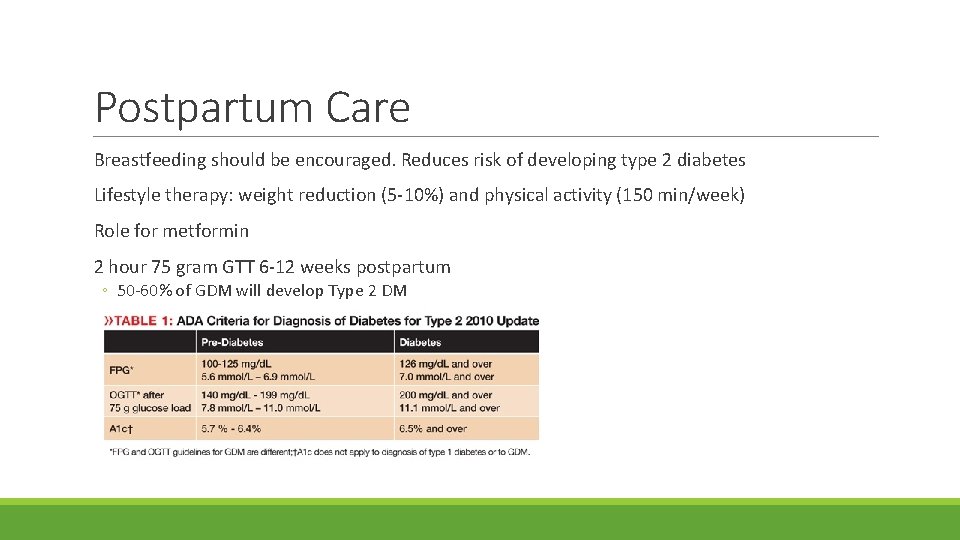
Postpartum Care Breastfeeding should be encouraged. Reduces risk of developing type 2 diabetes Lifestyle therapy: weight reduction (5 -10%) and physical activity (150 min/week) Role for metformin 2 hour 75 gram GTT 6 -12 weeks postpartum ◦ 50 -60% of GDM will develop Type 2 DM ◦ If normal, re-test every 3 years
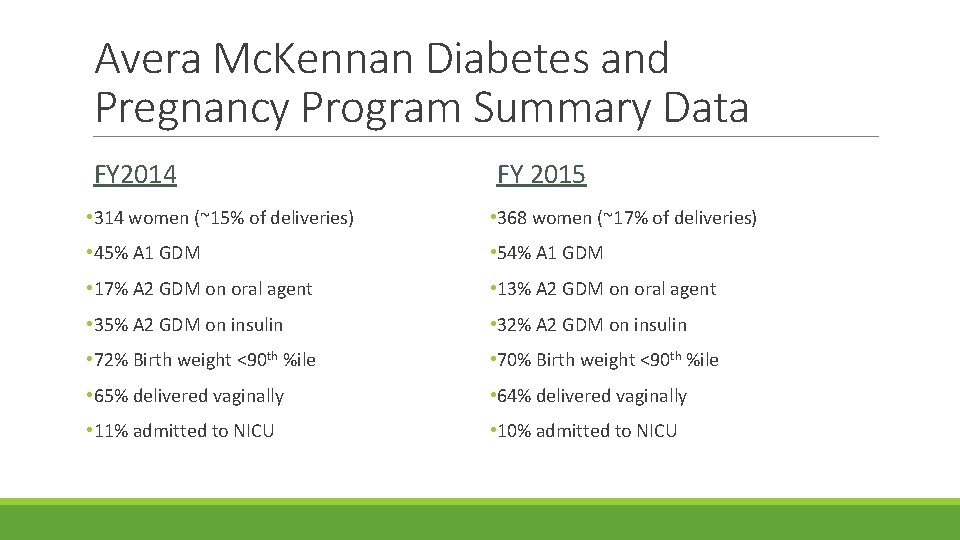
Avera Mc. Kennan Diabetes and Pregnancy Program Summary Data FY 2014 FY 2015 • 314 women (~15% of deliveries) • 368 women (~17% of deliveries) • 45% A 1 GDM • 54% A 1 GDM • 17% A 2 GDM on oral agent • 13% A 2 GDM on oral agent • 35% A 2 GDM on insulin • 32% A 2 GDM on insulin • 72% Birth weight <90 th %ile • 70% Birth weight <90 th %ile • 65% delivered vaginally • 64% delivered vaginally • 11% admitted to NICU • 10% admitted to NICU

References American College of Obstetricians and Gynecologists. Practice Bulletin No. 180: Gestational Diabetes Mellitus, July 2017 Management of Diabetes in Pregnancy. American Diabetes Association Clinical Practice Recommendations, Diabetes Care, January 2015 38: S 77 -S 79 International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, et. Diabetes Care 2010: 33: 676 Moyer VA, U. S. Preventive Services Task Force. Screening for gestational diabetes mellitus: U. S Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160: 414 HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991

Questions? ? Thank you!
- Slides: 39