Dia Mundial do Rim O Dia Mundial do














- Slides: 41


Dia Mundial do Rim • O Dia Mundial do Rim é celebrado na segunda quinta-feira de março • Objetivo: Sensibilizar a população em geral para as doenças renais que afetam milhões de pessoas em todo o mundo • A detecção precoce e a adoção de um estilo de vida saudável são fortes aliados para evitar o aumento do número de casos das doenças renais crônicas

O Dia Mundial do Rim foi criado em 2006. A cada ano é escolhido um tema particular • • • 2016 - Doença renal e as crianças e “Agir cedo para prevenir”. 2015 – Saúde Renal para Todos 2014 – A Doença Renal Crônica e o Envelhecimento 2013 – Rins para a Vida – Pare a Lesão Renal Aguda! 2012 – Doar – Rins para a Vida – Receber 2011 – Proteja os seus Rins: Salve o seu Coração 2010 – Proteja os seus Rins: Controle a Diabetes 2009 – Proteja os seus Rins: Controle a Pressão Arterial 2008 – Os Seus Rins Incríveis 2007 – Doença Renal Crônica: Comum, Nociva e Tratável 2006 – Os seus Rins estão OK?

“Homem Nu amanhece vestido”





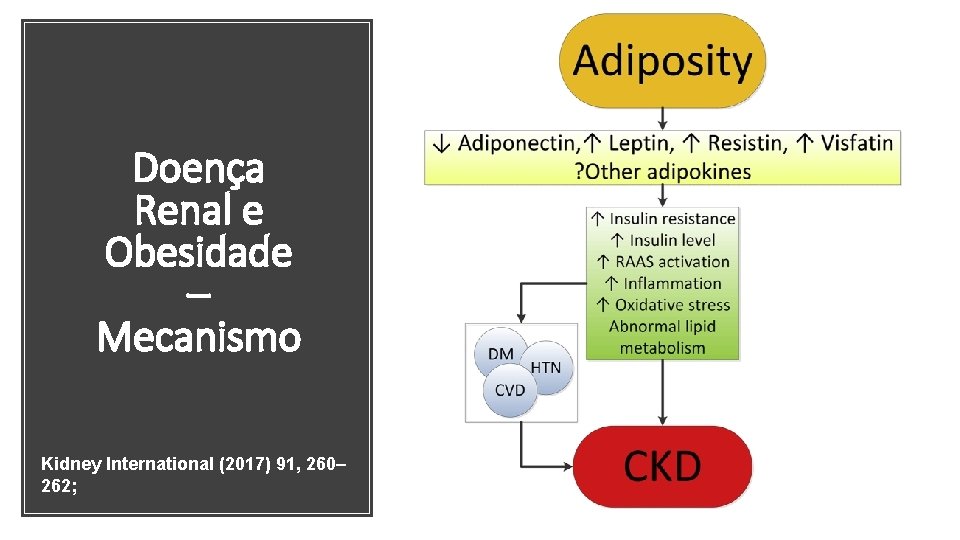
DRC e Obesidade • DRC afeta 1 em 10 pessoas em nível mundial. • Afeta cerca de 641 milhões de adultos (220 milhões – crianças em idade escolar) ou 13% da população mundial adulta e pode chegar até 20% em 2025

DRC e Obesidade • Obesidade aumenta o risco de fatores de risco para DRC, como DM e HAS. • Obesos possuem um risco 83% maior de DRC, comparados a indivíduos com peso normal. • Impacto direto na DRC e na ESRD • 2, 6 milhões de pacientes em diálise (2010), com projeção de 5, 4 milhões (2030) • A boa notícia é que a obesidade, bem como a DRC, é em grande parte evitável.

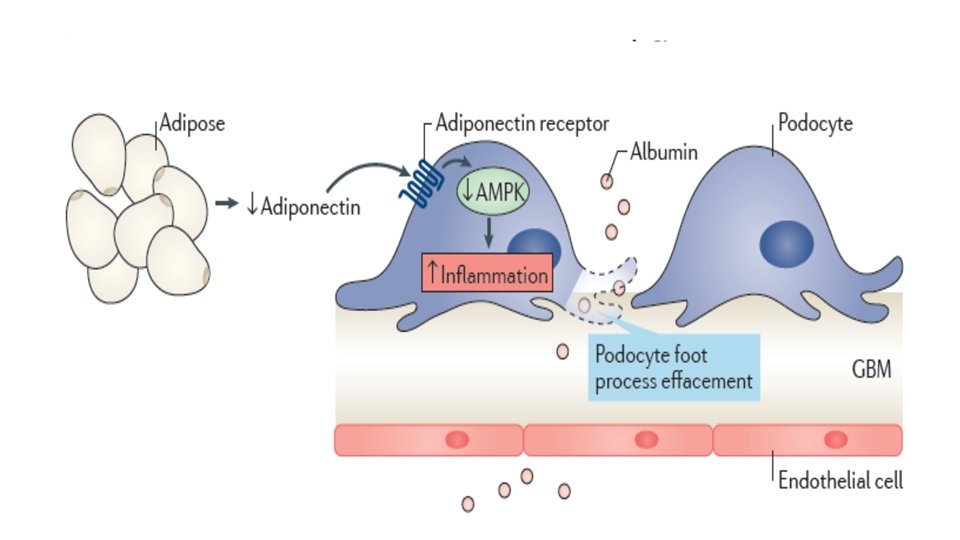
Doença Renal e Obesidade – Mecanismo Kidney International (2017) 91, 260– 262;



Kidney International 2017 91, 260 -262 DOI: (10. 1016/j. kint. 2016. 10. 019) Copyright © 2016 World Kidney Day 2017 Steering Committee Terms and Conditions

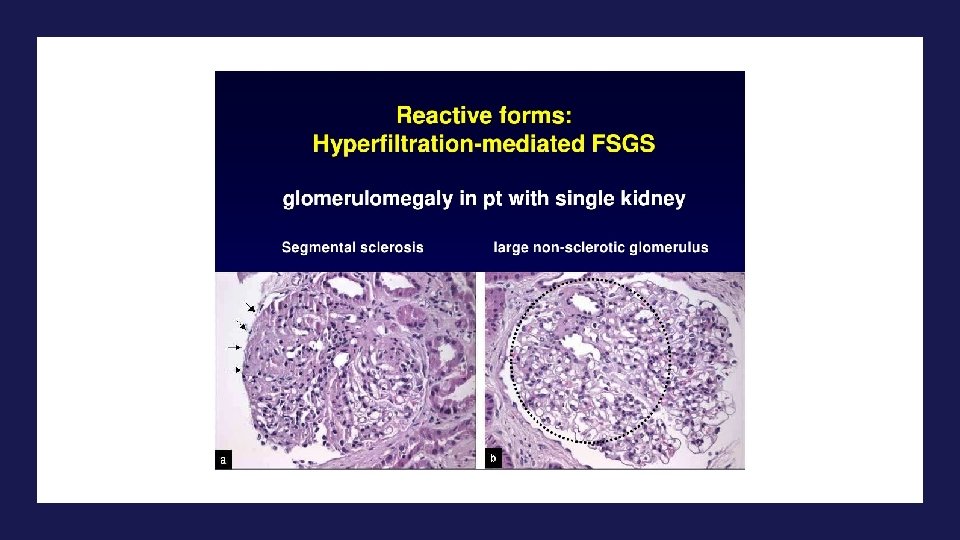
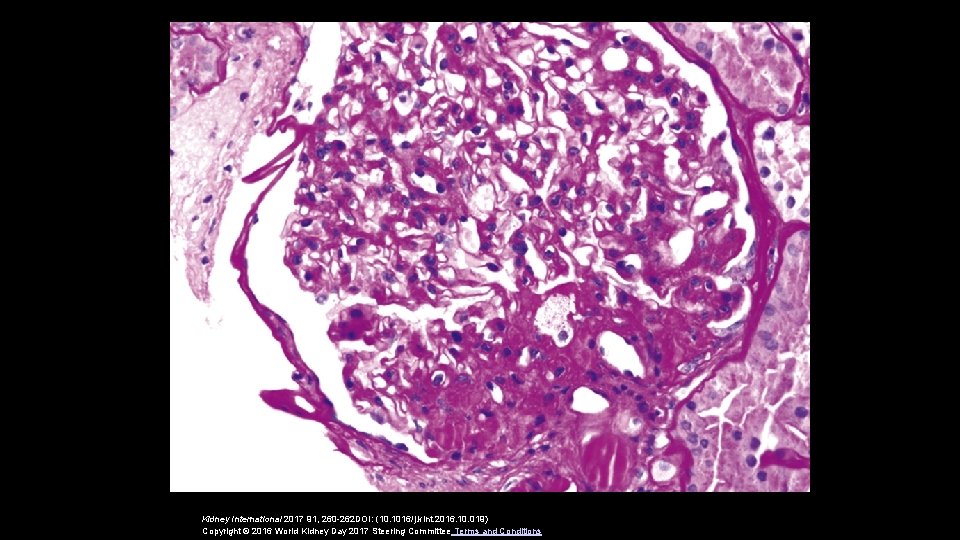
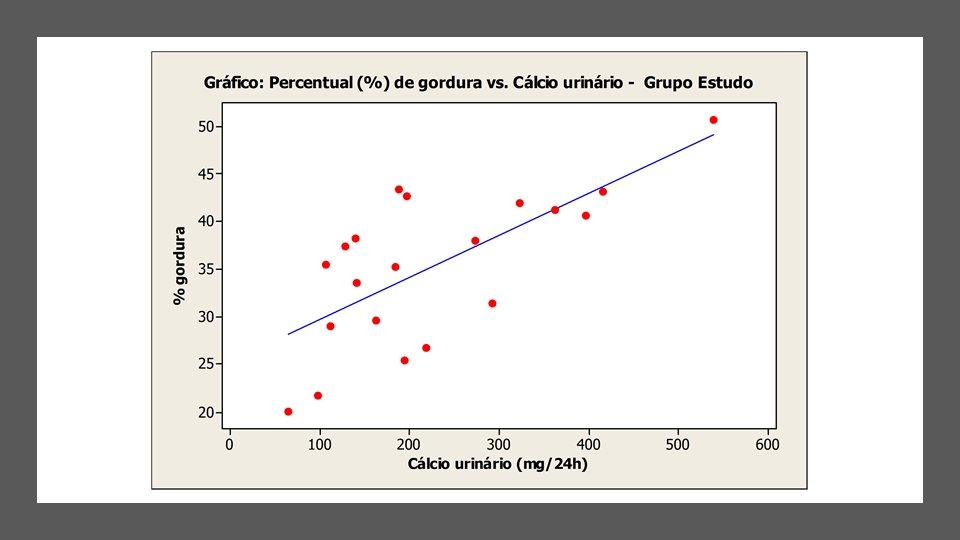
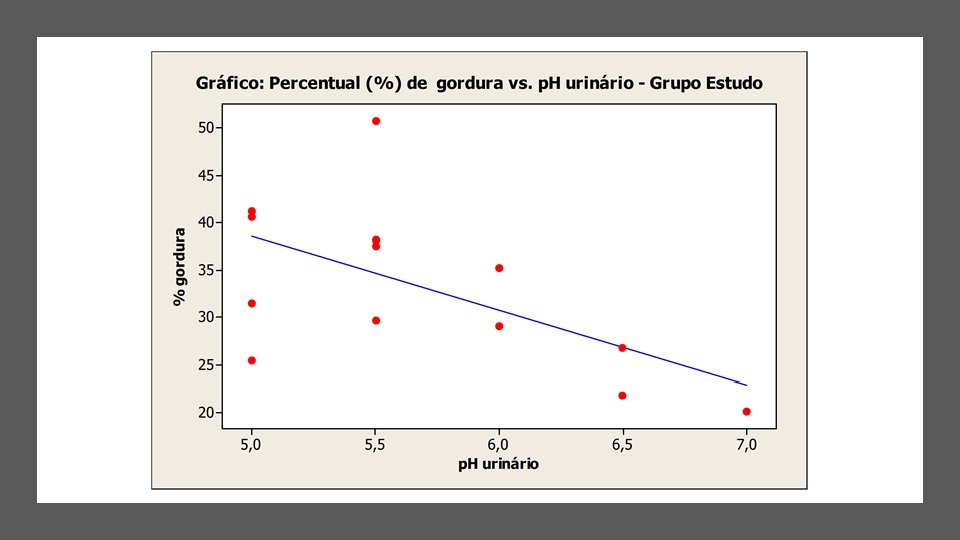
2 condições associadas à obesidade • Nefrolitíase • Neoplasias renais

SS Resistência à insulina

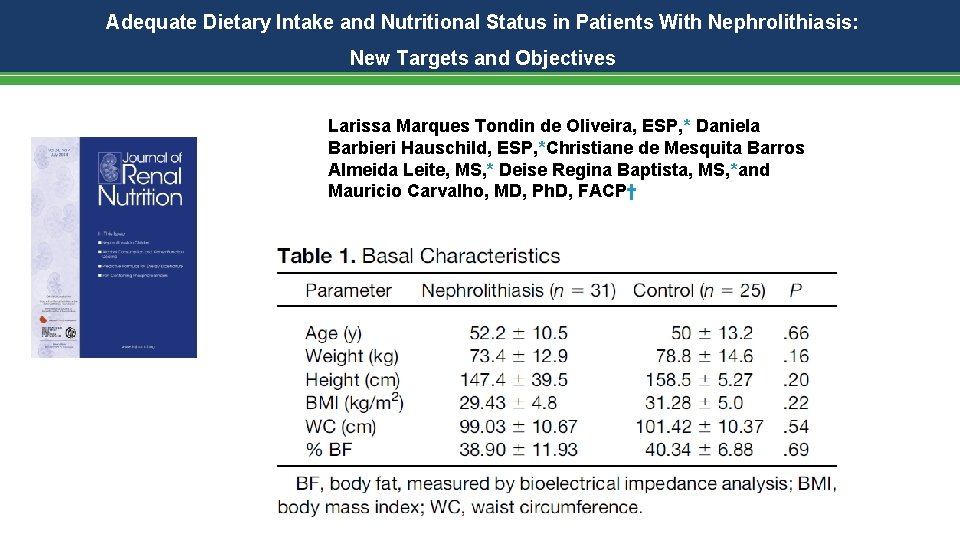
Adequate Dietary Intake and Nutritional Status in Patients With Nephrolithiasis: New Targets and Objectives Larissa Marques Tondin de Oliveira, ESP, * Daniela Barbieri Hauschild, ESP, *Christiane de Mesquita Barros Almeida Leite, MS, * Deise Regina Baptista, MS, *and Mauricio Carvalho, MD, Ph. D, FACP†

Adequate Dietary Intake and Nutritional Status in Patients With Nephrolithiasis: New Targets and Objectives Larissa Marques Tondin de Oliveira, ESP, * Daniela Barbieri Hauschild, ESP, *Christiane de Mesquita Barros Almeida Leite, MS, * Deise Regina Baptista, MS, *and Mauricio Carvalho, MD, Ph. D, FACP†



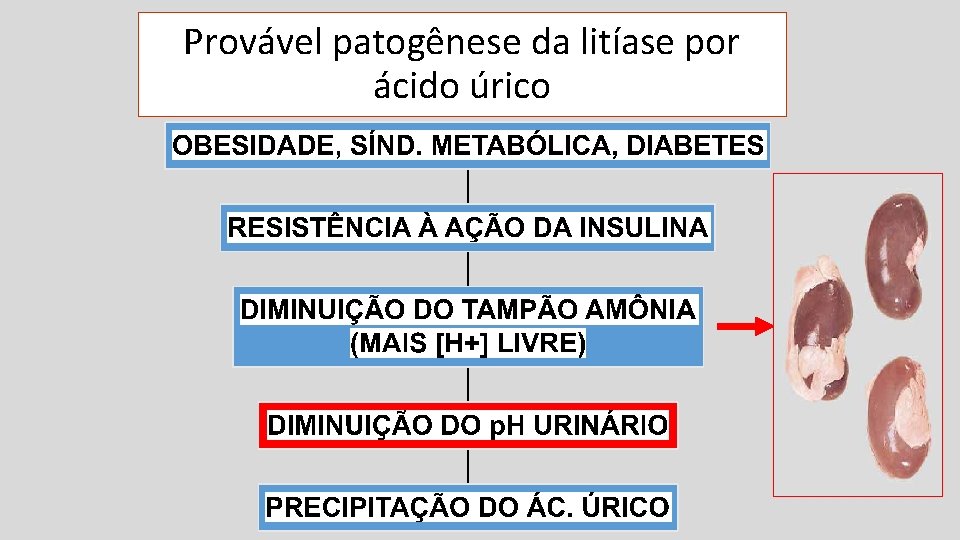
Provável patogênese da litíase por ácido úrico

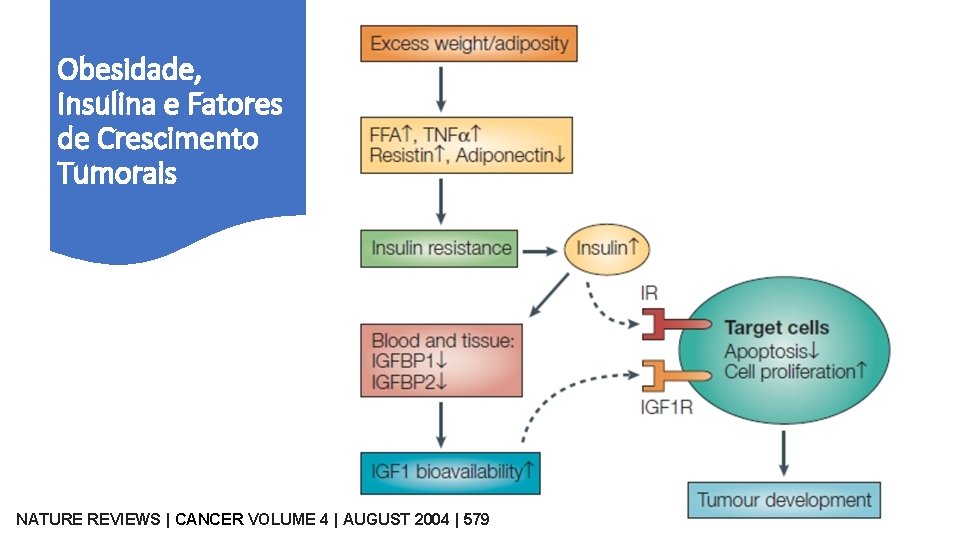
Obesidade, Insulina e Fatores de Crescimento Tumorais NATURE REVIEWS | CANCER VOLUME 4 | AUGUST 2004 | 579

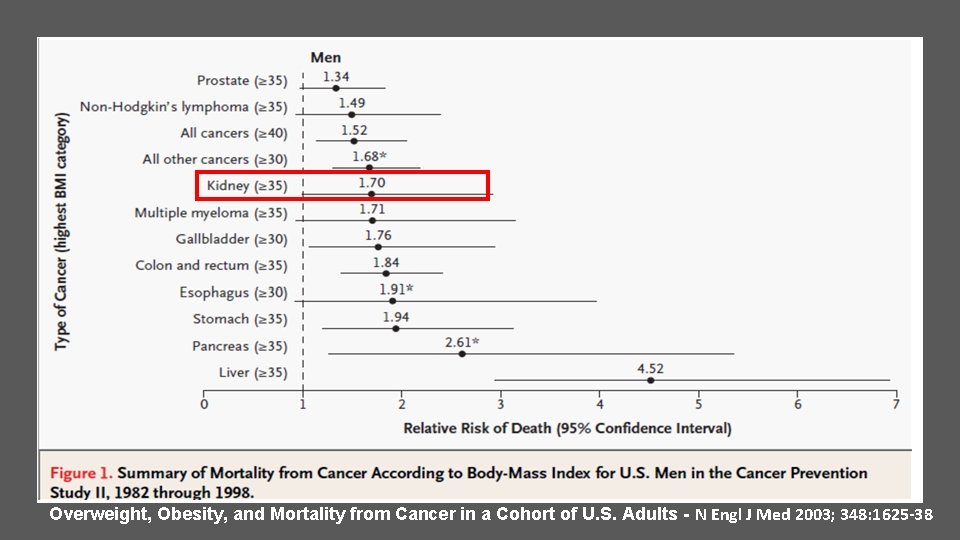
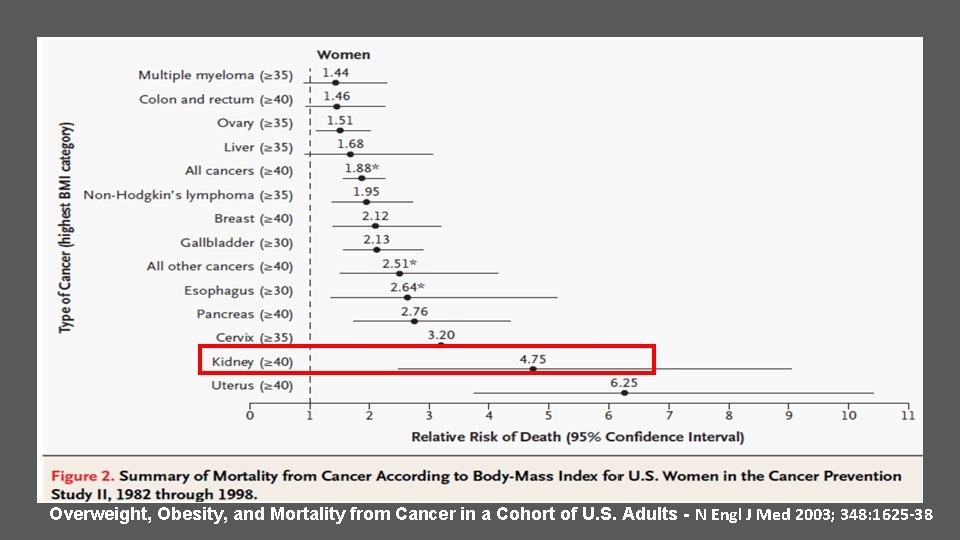
Overweight, Obesity, and Mortality from Cancer in a Cohort of U. S. Adults - N Engl J Med 2003; 348: 1625 -38

Overweight, Obesity, and Mortality from Cancer in a Cohort of U. S. Adults - N Engl J Med 2003; 348: 1625 -38

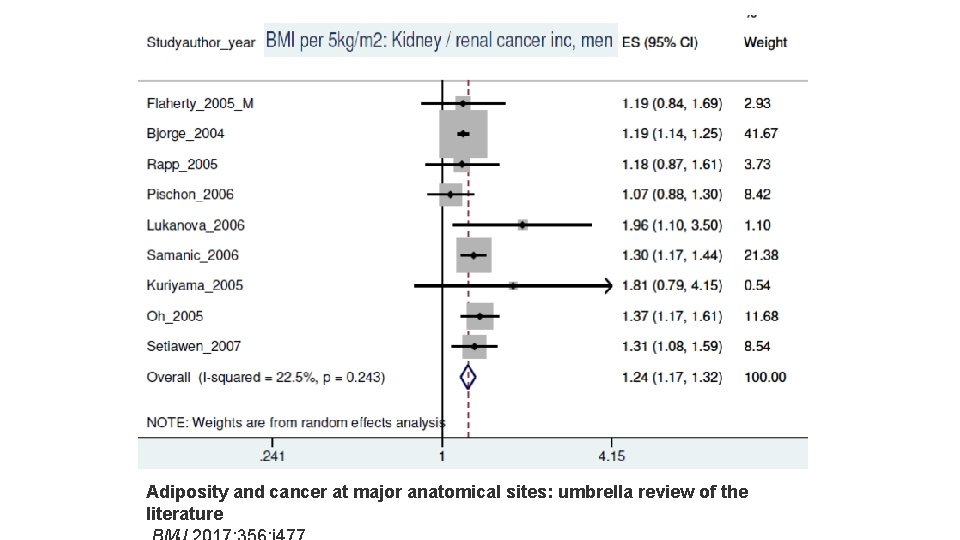
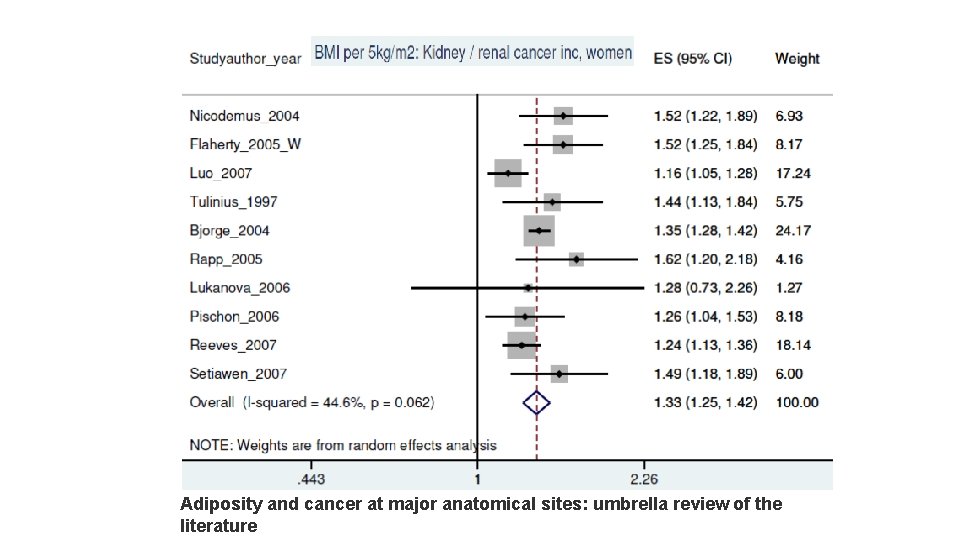
Adiposity and cancer at major anatomical sites: umbrella review of the literature

Adiposity and cancer at major anatomical sites: umbrella review of the literature

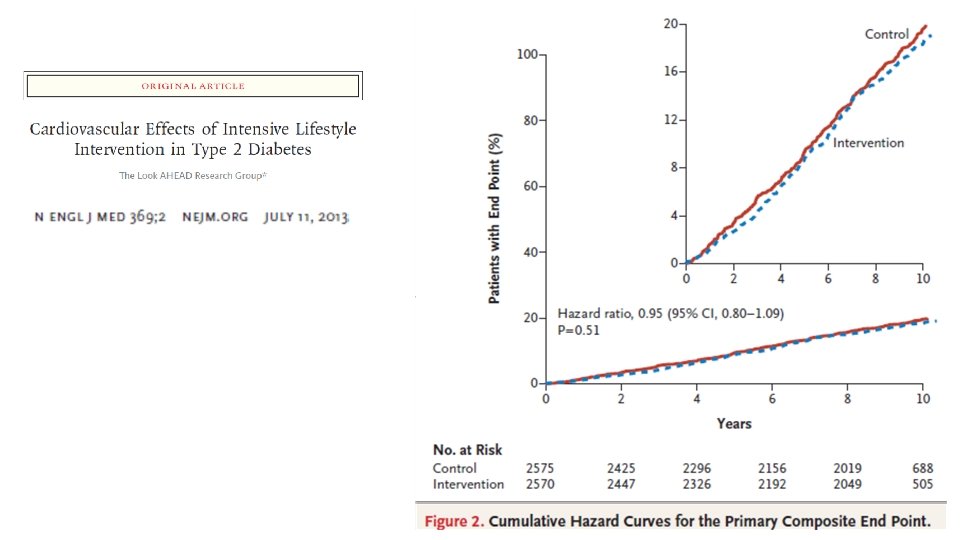
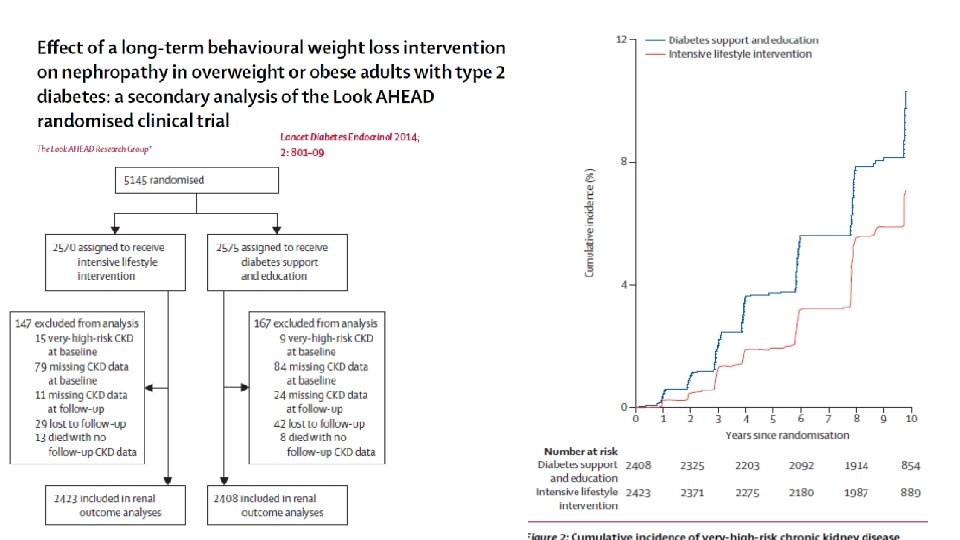
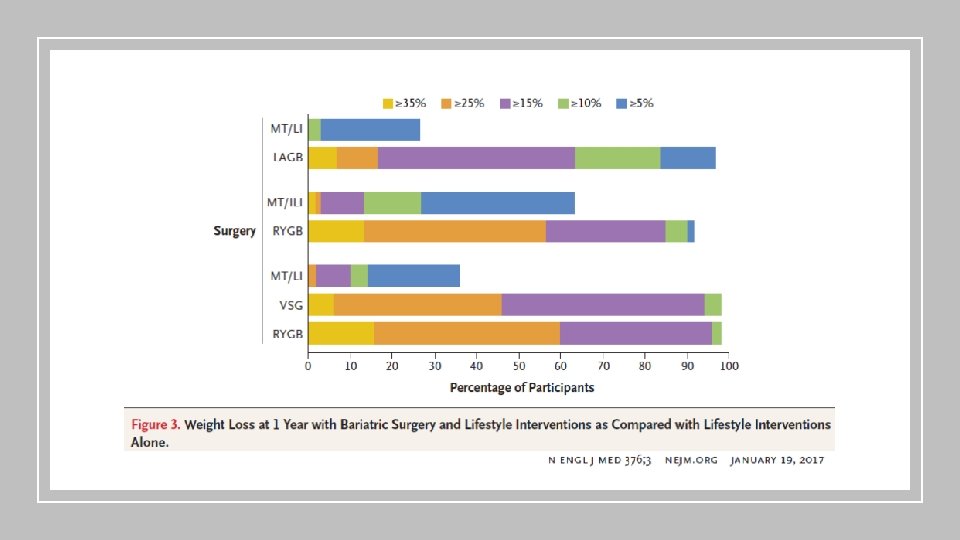
Estratégias de manejo • Modificação de Estilo de Vida - Look Ahead • Cirurgia Bariátrica • Farmacológico • Liraglutida – Estudo LEADER • Empaglifozina – Estudo EMPAREG




Clinical Outcomes with Antihyperglycemic Agents LEADER (LIRAGLUTIDE EFFECT AND ACTION IN DIABETES: EVALUATION OF CARDIOVASCULAR OUTCOME RESULTS) 31

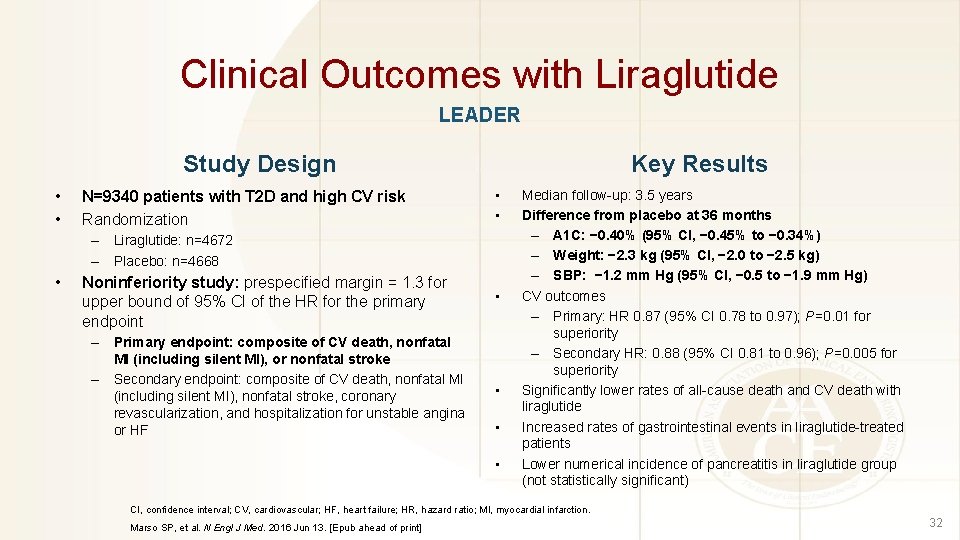
Clinical Outcomes with Liraglutide LEADER Study Design • • N=9340 patients with T 2 D and high CV risk Randomization Key Results • • – Liraglutide: n=4672 – Placebo: n=4668 • Noninferiority study: prespecified margin = 1. 3 for upper bound of 95% CI of the HR for the primary endpoint – Primary endpoint: composite of CV death, nonfatal MI (including silent MI), or nonfatal stroke – Secondary endpoint: composite of CV death, nonfatal MI (including silent MI), nonfatal stroke, coronary revascularization, and hospitalization for unstable angina or HF • • Median follow-up: 3. 5 years Difference from placebo at 36 months – A 1 C: − 0. 40% (95% CI, − 0. 45% to − 0. 34%) – Weight: − 2. 3 kg (95% CI, − 2. 0 to − 2. 5 kg) – SBP: − 1. 2 mm Hg (95% CI, − 0. 5 to − 1. 9 mm Hg) CV outcomes – Primary: HR 0. 87 (95% CI 0. 78 to 0. 97); P=0. 01 for superiority – Secondary HR: 0. 88 (95% CI 0. 81 to 0. 96); P=0. 005 for superiority Significantly lower rates of all-cause death and CV death with liraglutide Increased rates of gastrointestinal events in liraglutide-treated patients Lower numerical incidence of pancreatitis in liraglutide group (not statistically significant) CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MI, myocardial infarction. Marso SP, et al. N Engl J Med. 2016 Jun 13. [Epub ahead of print] 32

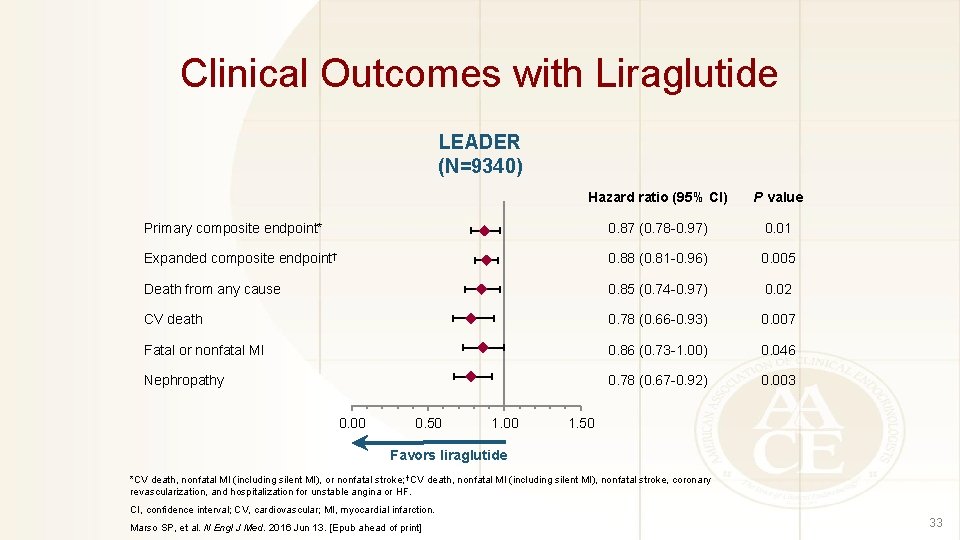
Clinical Outcomes with Liraglutide LEADER (N=9340) Hazard ratio (95% CI) P value Primary composite endpoint* 0. 87 (0. 78 -0. 97) 0. 01 Expanded composite endpoint† 0. 88 (0. 81 -0. 96) 0. 005 Death from any cause 0. 85 (0. 74 -0. 97) 0. 02 CV death 0. 78 (0. 66 -0. 93) 0. 007 Fatal or nonfatal MI 0. 86 (0. 73 -1. 00) 0. 046 Nephropathy 0. 78 (0. 67 -0. 92) 0. 003 0. 00 0. 50 1. 00 1. 50 Favors liraglutide *CV death, nonfatal MI (including silent MI), or nonfatal stroke; †CV death, nonfatal MI (including silent MI), nonfatal stroke, coronary revascularization, and hospitalization for unstable angina or HF. CI, confidence interval; CV, cardiovascular; MI, myocardial infarction. Marso SP, et al. N Engl J Med. 2016 Jun 13. [Epub ahead of print] 33

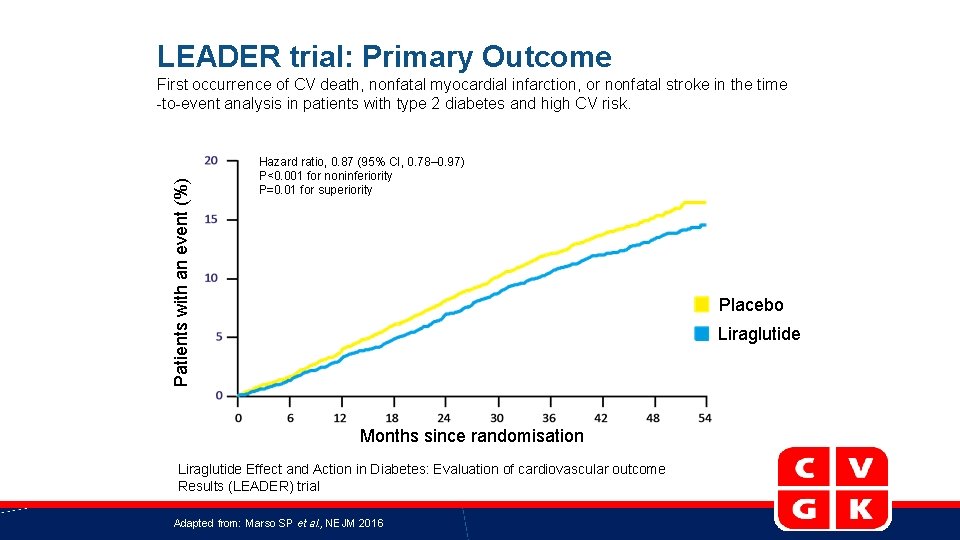
LEADER trial: Primary Outcome Patients with an event (%) First occurrence of CV death, nonfatal myocardial infarction, or nonfatal stroke in the time -to-event analysis in patients with type 2 diabetes and high CV risk. Hazard ratio, 0. 87 (95% CI, 0. 78– 0. 97) P<0. 001 for noninferiority P=0. 01 for superiority Placebo Liraglutide Months since randomisation Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial Adapted from: Marso SP et al. , NEJM 2016

Clinical Outcomes with Antihyperglycemic Agents EMPA-REG OUTCOME (EMPAGLIFLOZIN CARDIOVASCULAR OUTCOME EVENT TRIAL IN TYPE 2 DIABETES MELLITUS PATIENTS) 35

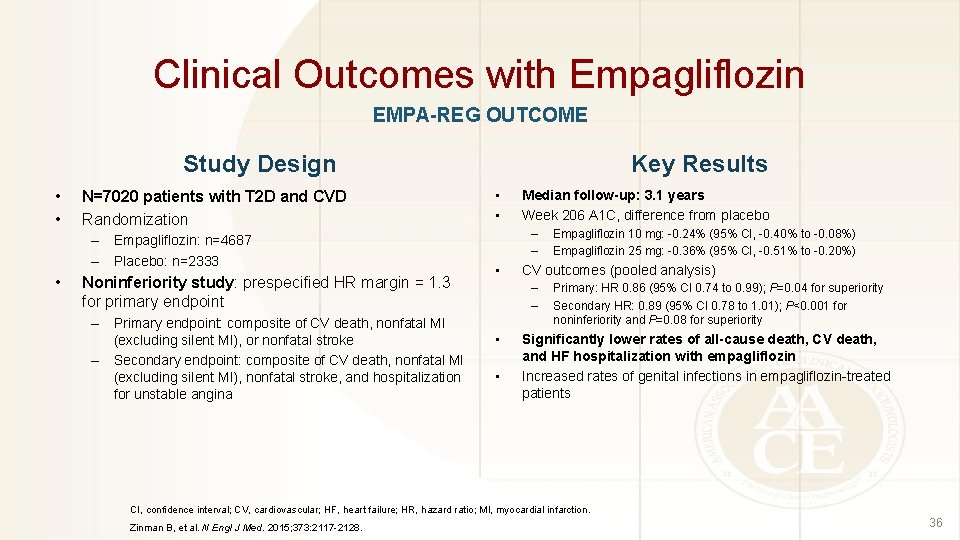
Clinical Outcomes with Empagliflozin EMPA-REG OUTCOME Study Design • • N=7020 patients with T 2 D and CVD Randomization – Empagliflozin: n=4687 – Placebo: n=2333 • Noninferiority study: prespecified HR margin = 1. 3 for primary endpoint – Primary endpoint: composite of CV death, nonfatal MI (excluding silent MI), or nonfatal stroke – Secondary endpoint: composite of CV death, nonfatal MI (excluding silent MI), nonfatal stroke, and hospitalization for unstable angina Key Results • • Median follow-up: 3. 1 years Week 206 A 1 C, difference from placebo – – • CV outcomes (pooled analysis) – – • • Empagliflozin 10 mg: -0. 24% (95% CI, -0. 40% to -0. 08%) Empagliflozin 25 mg: -0. 36% (95% CI, -0. 51% to -0. 20%) Primary: HR 0. 86 (95% CI 0. 74 to 0. 99); P=0. 04 for superiority Secondary HR: 0. 89 (95% CI 0. 78 to 1. 01); P<0. 001 for noninferiority and P=0. 08 for superiority Significantly lower rates of all-cause death, CV death, and HF hospitalization with empagliflozin Increased rates of genital infections in empagliflozin-treated patients CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MI, myocardial infarction. Zinman B, et al. N Engl J Med. 2015; 373: 2117 -2128. 36

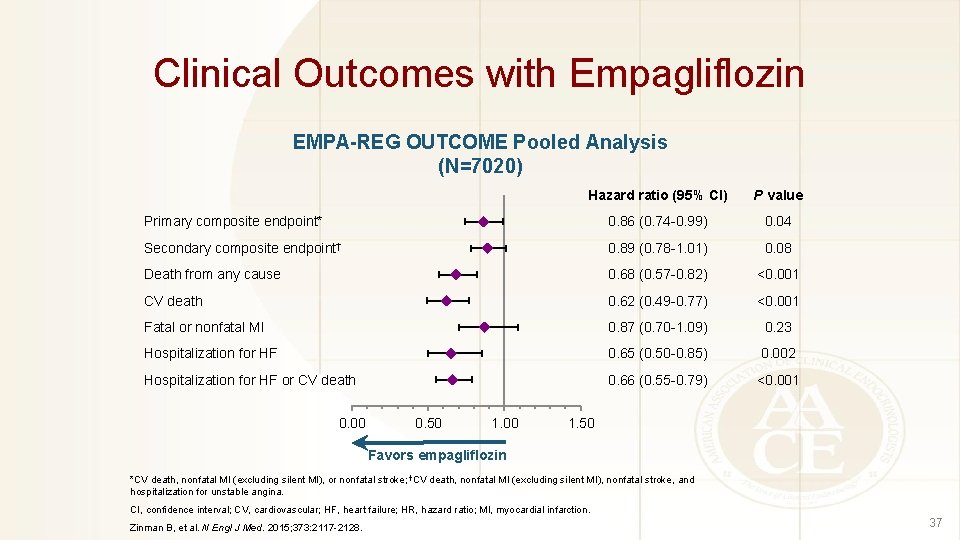
Clinical Outcomes with Empagliflozin EMPA-REG OUTCOME Pooled Analysis (N=7020) Hazard ratio (95% CI) P value Primary composite endpoint* 0. 86 (0. 74 -0. 99) 0. 04 Secondary composite endpoint† 0. 89 (0. 78 -1. 01) 0. 08 Death from any cause 0. 68 (0. 57 -0. 82) <0. 001 CV death 0. 62 (0. 49 -0. 77) <0. 001 Fatal or nonfatal MI 0. 87 (0. 70 -1. 09) 0. 23 Hospitalization for HF 0. 65 (0. 50 -0. 85) 0. 002 Hospitalization for HF or CV death 0. 66 (0. 55 -0. 79) <0. 001 0. 00 0. 50 1. 00 1. 50 Favors empagliflozin *CV death, nonfatal MI (excluding silent MI), or nonfatal stroke; †CV death, nonfatal MI (excluding silent MI), nonfatal stroke, and hospitalization for unstable angina. CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MI, myocardial infarction. Zinman B, et al. N Engl J Med. 2015; 373: 2117 -2128. 37

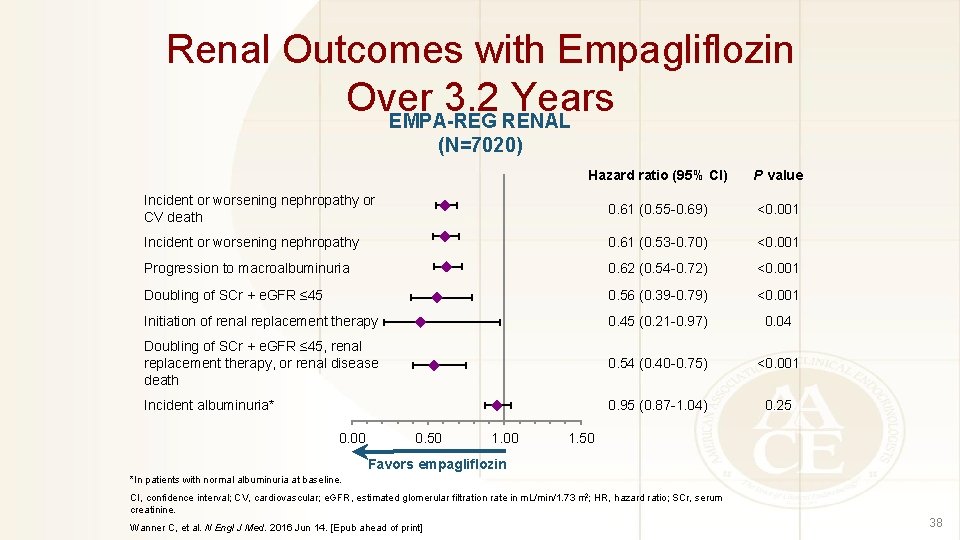
Renal Outcomes with Empagliflozin Over 3. 2 Years EMPA-REG RENAL (N=7020) Hazard ratio (95% CI) P value Incident or worsening nephropathy or CV death 0. 61 (0. 55 -0. 69) <0. 001 Incident or worsening nephropathy 0. 61 (0. 53 -0. 70) <0. 001 Progression to macroalbuminuria 0. 62 (0. 54 -0. 72) <0. 001 Doubling of SCr + e. GFR ≤ 45 0. 56 (0. 39 -0. 79) <0. 001 Initiation of renal replacement therapy 0. 45 (0. 21 -0. 97) 0. 04 Doubling of SCr + e. GFR ≤ 45, renal replacement therapy, or renal disease death 0. 54 (0. 40 -0. 75) <0. 001 Incident albuminuria* 0. 95 (0. 87 -1. 04) 0. 25 0. 00 0. 50 1. 00 1. 50 Favors empagliflozin *In patients with normal albuminuria at baseline. CI, confidence interval; CV, cardiovascular; e. GFR, estimated glomerular filtration rate in m. L/min/1. 73 m 2; HR, hazard ratio; SCr, serum creatinine. Wanner C, et al. N Engl J Med. 2016 Jun 14. [Epub ahead of print] 38

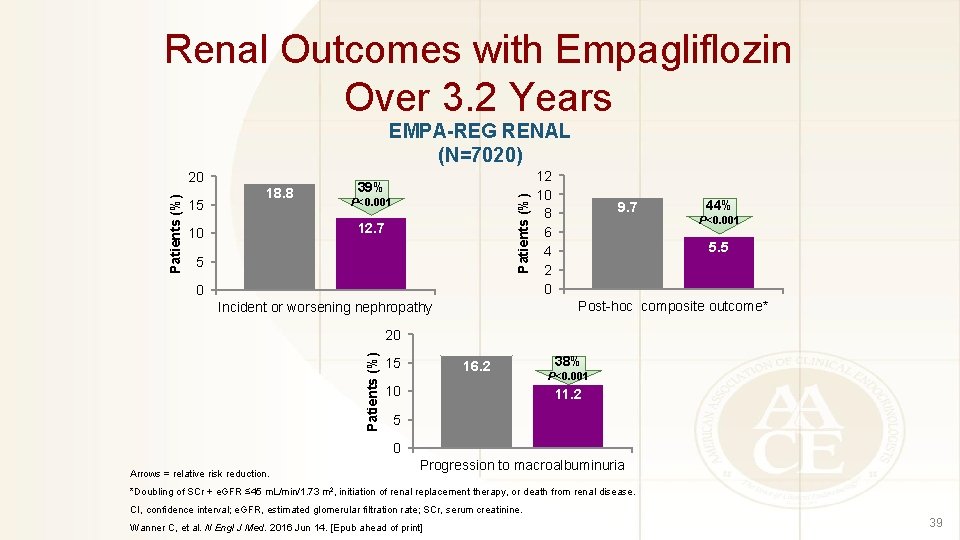
Renal Outcomes with Empagliflozin Over 3. 2 Years EMPA-REG RENAL (N=7020) 15 18. 8 39% Patients (%) 20 P<0. 001 12. 7 10 5 0 12 10 8 6 4 2 0 9. 7 44% P<0. 001 5. 5 Post-hoc composite outcome* Incident or worsening nephropathy Patients (%) 20 15 16. 2 10 38% P<0. 001 11. 2 5 0 Arrows = relative risk reduction. Progression to macroalbuminuria *Doubling of SCr + e. GFR ≤ 45 m. L/min/1. 73 m 2, initiation of renal replacement therapy, or death from renal disease. CI, confidence interval; e. GFR, estimated glomerular filtration rate; SCr, serum creatinine. Wanner C, et al. N Engl J Med. 2016 Jun 14. [Epub ahead of print] 39

Em resumo… 1. DRC – 1 em 10 indivíduos; Obesidade – 50% população brasileira 2. Consequências: a) Glomerulopatia da Obesidade b) Nefrolitíase c) Neoplasia renal 3. Tratamento: MEV; Bariátrica; Novos agentes (Análogos GLP -1; SGLT 2) 4. Obesidade, bem como a DRC, é em grande parte evitável 40
