Device Review Decision Tree Version Date 42815 This
- Slides: 1

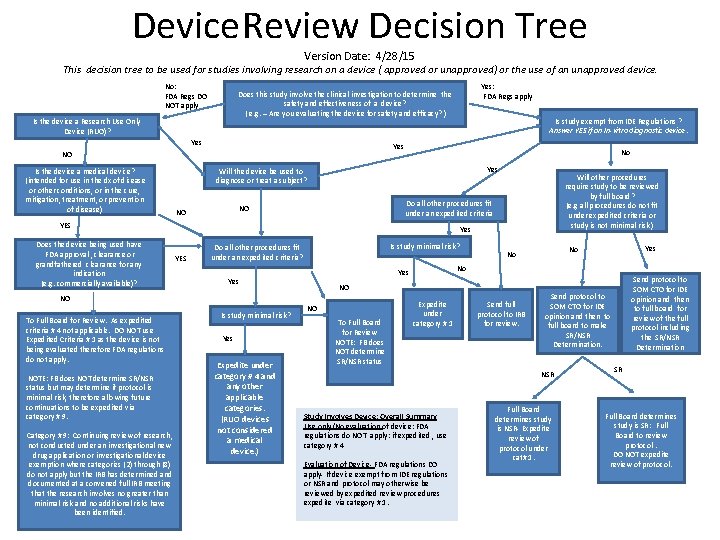
Device Review Decision Tree Version Date: 4/28/15 This decision tree to be used for studies involving research on a device ( approved or unapproved) or the use of an unapproved device. No: FDA Regs DO NOT apply Is the device a Research Use Only Device (RUO)? Yes Is study exempt from IDE Regulations ? Answer YES if an In-vitro diagnostic device. Yes NO Is the device a medical device? (intended for use in the dx of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease) Yes: FDA Regs apply Does this study involve the clinical investigation to determine the safety and effectiveness of a device? ( e. g. – Are you evaluating the device for safety and efficacy? ) No Yes Will the device be used to diagnose or treat a subject? Do all other procedures fit under an expedited criteria NO NO YES Does the device being used have FDA approval , clearance or grandfathered clearance for any indication (e. g. commercially available)? Yes YES Is study minimal risk? Do all other procedures fit under an expedited criteria? NO NOTE: FB does NOT determine SR/NSR status but may determine if protocol is minimal risk, therefore allowing future continuations to be expedited via category # 9. Category # 9: Continuing review of research, not conducted under an investigational new drug application or investigational device exemption where categories (2) through (8) do not apply but the IRB has determined and documented at a convened full IRB meeting that the research involves no greater than minimal risk and no additional risks have been identified. Is study minimal risk? Yes Expedite under category # 4 and any other applicable categories. (RUO devices not considered a medical device. ) NO To Full Board for Review NOTE: FB does NOT determine SR/NSR status Yes No No No Yes NO To Full Board for Review. As expedited criteria # 4 not applicable. DO NOT use Expedited Criteria # 1 as the device is not being evaluated therefore FDA regulations do not apply. Will other procedures require study to be reviewed by full board? (e. g all procedures do not fit under expedited criteria or study is not minimal risk ) Expedite under category # 1 Send full protocol to IRB for review. Send protocol to SOM CTO for IDE opinion and then to full board to make SR/NSR Determination. NSR Study Involves Device: Overall Summary Use only/No evaluation of device: FDA regulations do NOT apply: if expedited , use category # 4 Evaluation of Device- FDA regulations DO apply- If device exempt from IDE regulations or NSR and protocol may otherwise be reviewed by expedited review procedures expedite via category # 1. Send protocol to SOM CTO for IDE opinion and then to full board for review of the full protocol including the SR/NSR Determination Full Board determines study is NSR- Expedite review of protocol under cat# 1. SR Full Board determines study is SR: Full Board to review protocol. DO NOT expedite review of protocol.