Development of the Periodic Table Chemistry 5A Development
Development of the Periodic Table Chemistry 5(A)
Development of the Periodic Table Learning objectives • Know the history of the periodic table • Understand how chemical and physical properties of the elements led to the development of the periodic table
Early Elements • Some elements have been known since ancient times – – – Copper (Cu) Gold (Au) Lead (Pb) Mercury (Hg) Silver (Ag) Tin (Sn) Mercury

Next Wave of Discovery • Limited ability to perform scientific research caused no new elements to be discovered for thousands of years • Scientific experimentation increased during the Enlightenment, additional elements were discovered • First chemistry textbook was published in the 1780 s by Lavoisier and listed the 23 elements known at that time http: //www. schuster-ingolstadt. de/Chemie. htm [public domain]
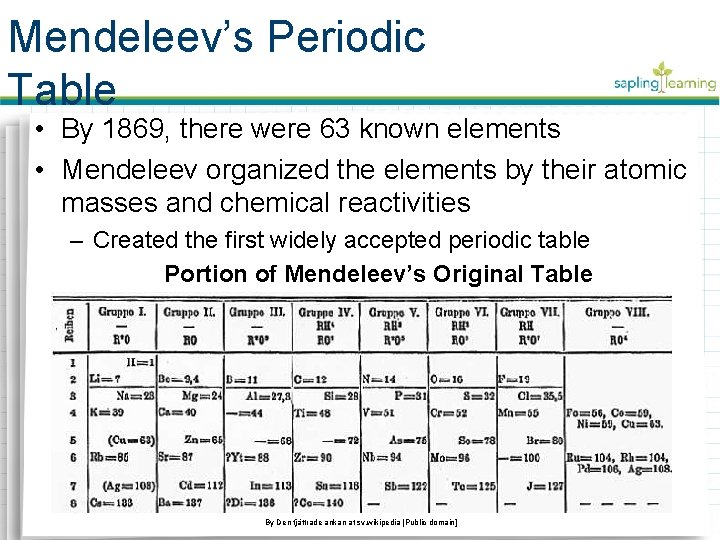
Mendeleev’s Periodic Table • By 1869, there were 63 known elements • Mendeleev organized the elements by their atomic masses and chemical reactivities – Created the first widely accepted periodic table Portion of Mendeleev’s Original Table By Den fjättrade ankan at sv. wikipedia [Public domain]

Patterns in Mendeleev’s Table • Mendeleev found patterns by arranging cards with the names, masses, and properties of elements on them • Grouped elements that had similar “combining powers” • Alkali metals had a combining power of one – Group I • Alkaline earth metals had combining power of two – Group II • Copper and mercury were difficult to classify since they combined in multiple ways – Now classified as transition metals • Hydrogen was difficult to classify since it had properties of the alkali metals and the halogens – Still difficult to place on modern periodic tables
Mendeleev’s Insights • To arrange elements, Mendeleev relied more on similarities in chemical and physical properties than accepted masses – Exceptions to arrangement by mass • Argon and potassium • Cobalt and nickel • Tellurium and iodine • Left spaces for undiscovered elements – Correctly predicted their chemical reactivities and masses • Gallium, scandium, and germanium
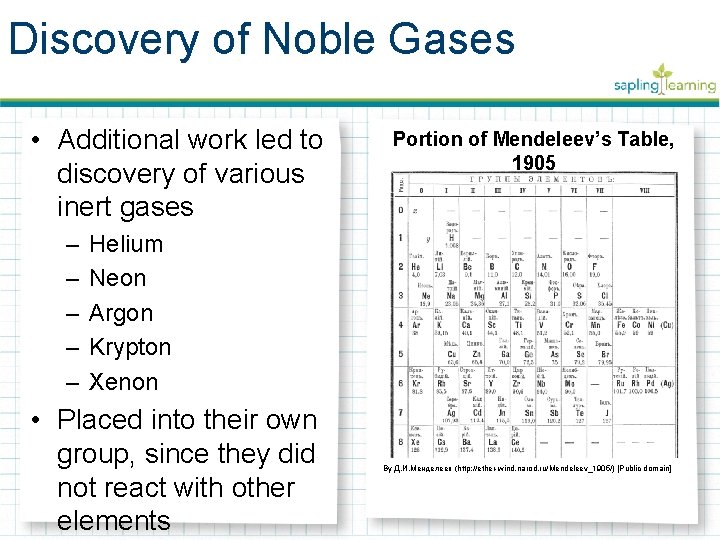
Discovery of Noble Gases • Additional work led to discovery of various inert gases – – – Portion of Mendeleev’s Table, 1905 Helium Neon Argon Krypton Xenon • Placed into their own group, since they did not react with other elements By Д. И. Менделеев (http: //ether-wind. narod. ru/Mendeleev_1905/) [Public domain]
Arranging Elements by Atomic Number • In 1914, Henry Moseley’s work with x-rays showed each element had a unique positive charge in the nucleus – Atomic number – number of protons in an atom • When Mendeleev’s table was organized by atomic number, problems caused by organization by mass disappeared • Moseley’s atomic numbers confirmed the existence of predicted elements technetium and promethium • Arranging by atomic number also eliminated problems associated with isotopes of the same element having different atomic masses
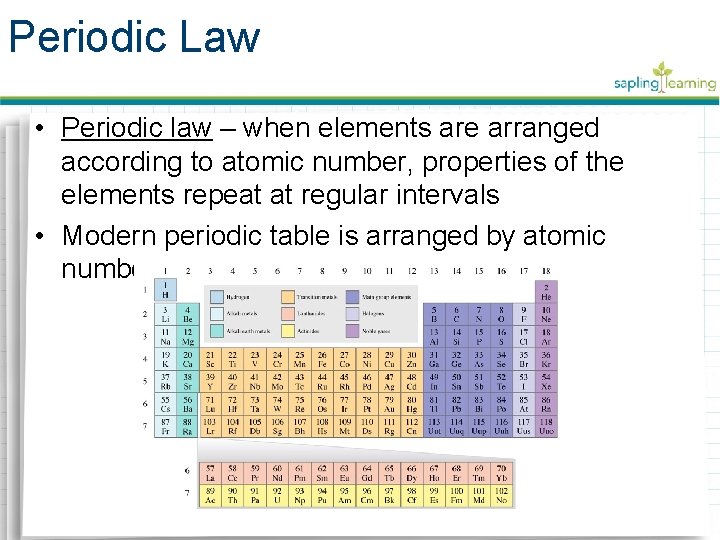
Periodic Law • Periodic law – when elements are arranged according to atomic number, properties of the elements repeat at regular intervals • Modern periodic table is arranged by atomic number
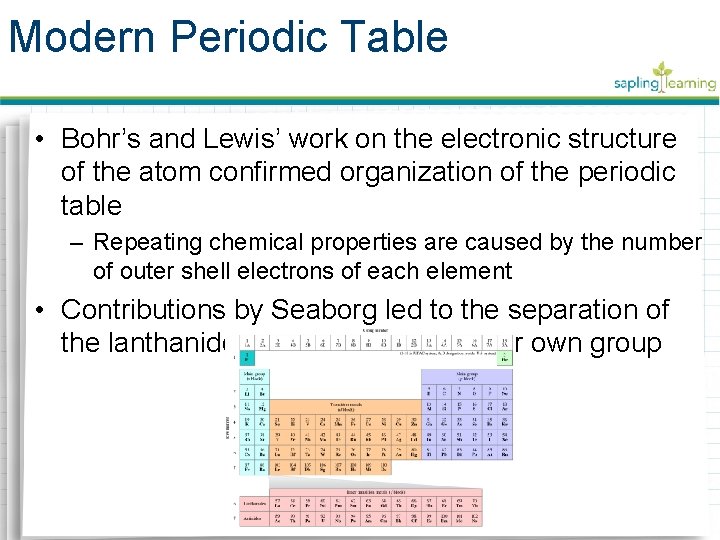
Modern Periodic Table • Bohr’s and Lewis’ work on the electronic structure of the atom confirmed organization of the periodic table – Repeating chemical properties are caused by the number of outer shell electrons of each element • Contributions by Seaborg led to the separation of the lanthanides and actinides as their own group
Development of the Periodic Table Learning objectives • Know the history of the periodic table • Understand how chemical and physical properties of the elements led to the development of the periodic table
- Slides: 12