Development of the periodic table Ancient periodic table

Development of the periodic table

Ancient periodic table • The periodic table did not always look like this • Dimitri Mendeleev, a Russian chemist is said to be the father of the periodic table • Mendeleev was working at the same time as Julius Mayer, a German physicist and they both developed a similar periodic table even though they worked independently in 1869

How did Mendeleev do it? • He grouped elements together based on similar properties on their reactivity • He grouped them based on their reactions with oxygen • They went in different groups if they produced – XO – X 2 O – XO 2 – X 2 O 3
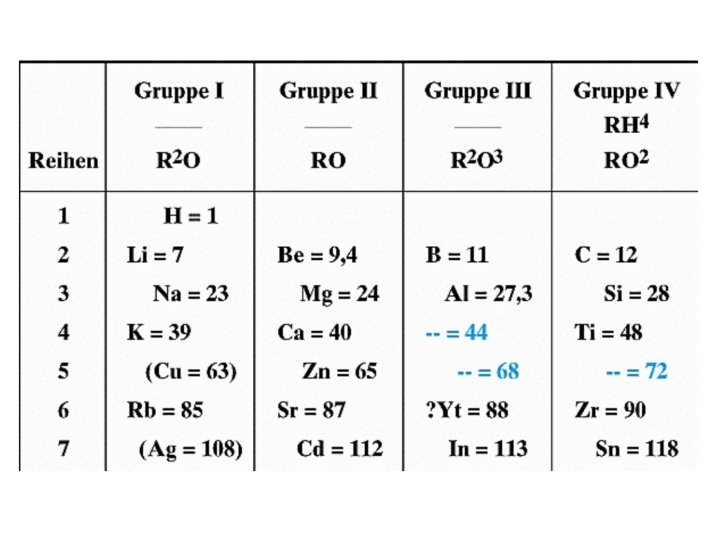

Periodic Law • Mendeleev said – “The properties of elements recur “periodically” when the elements are arranged in increasing order by their atomic weight” • This is where we get the word periodic from • Mendeleev also ordered his elements by atomic weight/mass
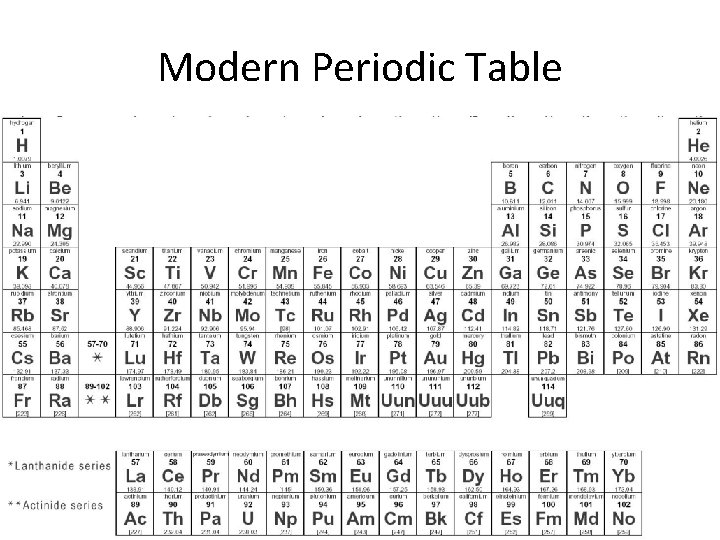
Modern Periodic Table

Modern Periodic Table • Elements are arranged by atomic number instead of mass • Elements are also grouped by their similar properties, not their reaction to water – We call these family

Family

Family • Families all share similar properties and allow us to predict their behaviour – Alkali Metals – group 1 – Alkaline Earth Metals – group 2 – Halogens – group 17 – Noble Gases – group 18

Homework • Page 160 #30
- Slides: 10