Development of the Atomic Theory Democritus was a








- Slides: 14

Development of the Atomic Theory Democritus was a Greek philosopher who theorized that all matter was made of invisible particles called atoms. Democritus of Abdera, about 460 -370 BCE © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

Development of the Atomic Theory His views contrasted those of Aristotle, who believed in the four elements; earth, water, air, fire. © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

Development of the Atomic Theory Most of our knowledge of Democritus comes from negative remarks about him in others’ writings. © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

Development of the Atomic Theory John Dalton 1766 -1844 © Fall 2005, Pflugerville ISD, 8 th Grade Dalton, a British chemist and teacher, did studies and experiments in weather, colorblindness, and gases. Unit A : Chapter 2

Development of the Atomic Theory He noticed that elements combine in specific proportions to form compounds, and theorized that their atoms combine at the same proportions © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

Development of the Atomic Theory Thomson’s experiments using a cathode-ray tube showed that smaller particles make up atoms Joseph John “J. J. ” Thomson 1856 -1940 © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

Development of the Atomic Theory Thomson received the Nobel Prize in physics in 1906 for his discovery of the electron. © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

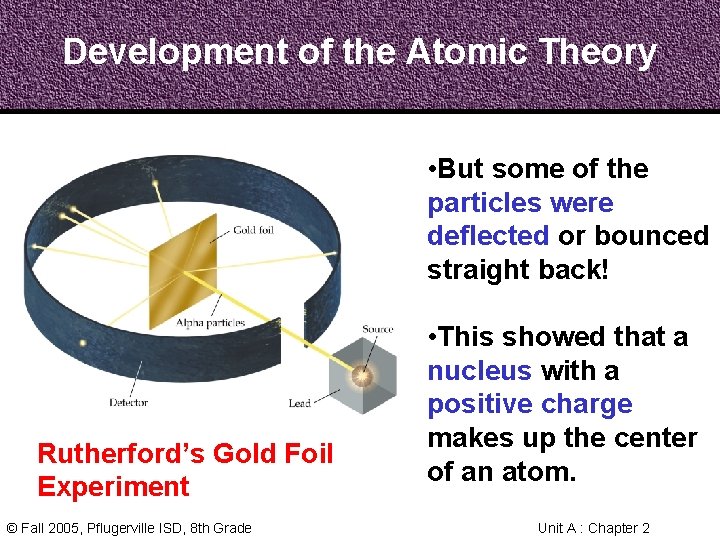
Development of the Atomic Theory Rutherford, a former student of Thomson’s from New Zealand, tested his teacher’s theories in his Gold Foil Experiment. Ernest Rutherford 1871 - 1937 © Fall 2005, Pflugerville ISD, 8 th Grade • He expected his alpha particles to go straight through the foil, and most of them did. Unit A : Chapter 2

Development of the Atomic Theory • But some of the particles were deflected or bounced straight back! Rutherford’s Gold Foil Experiment © Fall 2005, Pflugerville ISD, 8 th Grade • This showed that a nucleus with a positive charge makes up the center of an atom. Unit A : Chapter 2

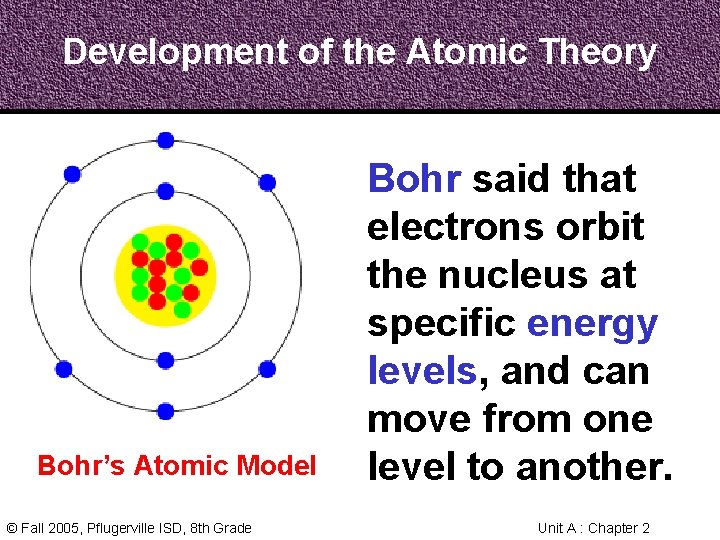
Development of the Atomic Theory Bohr, a Danish scientist who worked with Rutherford, described the motion of electrons around the nucleus. Niels Bohr 1885 -1962 © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

Development of the Atomic Theory Bohr’s Atomic Model © Fall 2005, Pflugerville ISD, 8 th Grade Bohr said that electrons orbit the nucleus at specific energy levels, and can move from one level to another. Unit A : Chapter 2

Development of the Atomic Theory To do this, Bohr said, the electrons must absorb or release energy, often in the form of light. © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

Development of the Atomic Theory Erwin Schrödinger and Werner Heisenberg’s work with the uncertainty principle explained that electrons do not travel in orbits. In fact, the exact path of a moving electron Schrödinger & Heisenberg cannot be predicted. © Fall 2005, Pflugerville ISD, 8 th Grade Unit A : Chapter 2

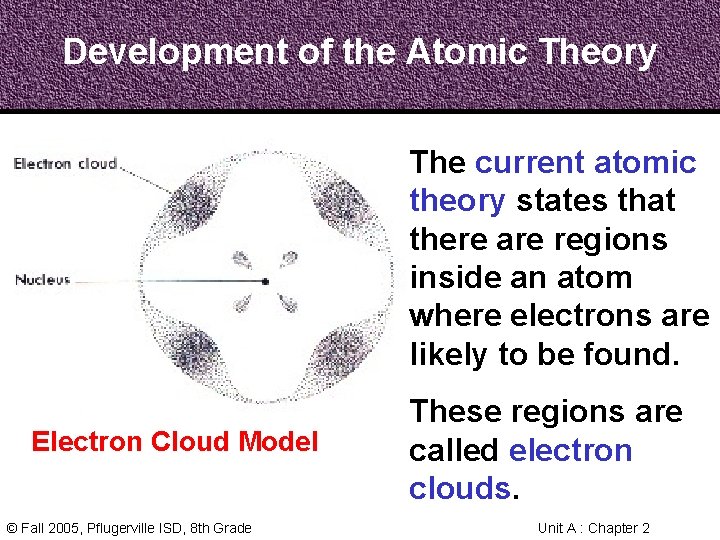
Development of the Atomic Theory The current atomic theory states that there are regions inside an atom where electrons are likely to be found. Electron Cloud Model © Fall 2005, Pflugerville ISD, 8 th Grade These regions are called electron clouds. Unit A : Chapter 2