Development of the Atomic structure Greek Model To








- Slides: 38

Development of the Atomic structure

Greek Model “To understand the very large, we must understand the very small. ” Democritus • Greek philosopher • Idea of ‘democracy’ • Idea of ‘atomos’ • Atomos = ‘indivisible’ • ‘Atom’ is derived • No experiments to support idea • Continuous vs. discontinuous theory of matter Democritus’s model of atom No protons, electrons, or neutrons Solid and INDESTRUCTABLE

Democritus DEMOCRITUS (400 BC) – First Atomic Hypothesis Atomos: Greek for “uncuttable”. Chop up a piece of matter until you reach the atomos. Properties of atoms: • indestructible. • changeable, however, into different forms. • an infinite number of kinds so there an infinite number of elements. • hard substances have rough, prickly atoms that stick together. • liquids have round, smooth atoms that slide over one another. • smell is caused by atoms interacting with the nose – rough atoms hurt. • sleep is caused by atoms escaping the brain. • death – too many escaped or didn’t return. • the heart is the center of anger. • the brain is the center of thought. • the liver is the seat of desire. “Nothing exists but atoms and space, all else is opinion”.

Dalton Model of the Atom Late 1700’s - John Dalton- England Teacher- summarized results of his experiments and those of others Combined ideas of elements with that of atoms in Dalton’s Atomic Theory

The Atomic Theory of Matter • In 1803, Dalton proposed that elements consist of individual particles called atoms. • His atomic theory of matter contains four hypotheses: 1. All matter is composed of tiny particles called atoms. 2. All atoms of an element are identical in mass and fundamental chemical properties. 3. A chemical compound is a substance that always contains the same atoms in the same ratio. 4. In chemical reactions, atoms from one or more compounds or elements redistribute or rearrange in relation to other atoms to form one or more new compounds. Atoms themselves do not undergo a change of identity in chemical reactions. Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.

Foundations of Atomic Theory Law of Conservation of Mass is neither destroyed nor created during ordinary chemical reactions. Law of Definite Proportions The fact that a chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. Law of Multiple Proportions If two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first elements is always a ratio of small whole numbers.

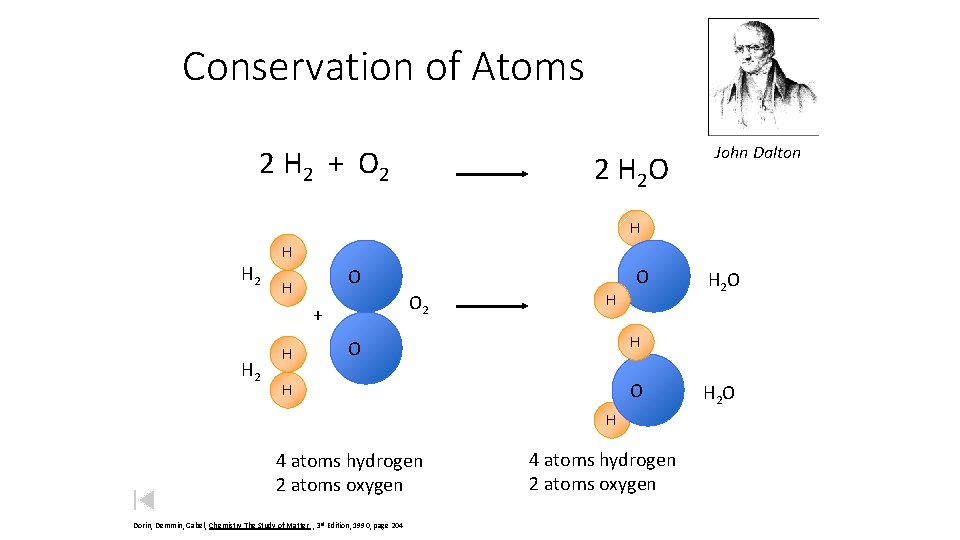
Conservation of Atoms 2 H 2 + O 2 2 H 2 O John Dalton H H 2 H O 2 + H 2 H O H H O O H H 4 atoms hydrogen 2 atoms oxygen Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 204 H 2 O 4 atoms hydrogen 2 atoms oxygen H 2 O

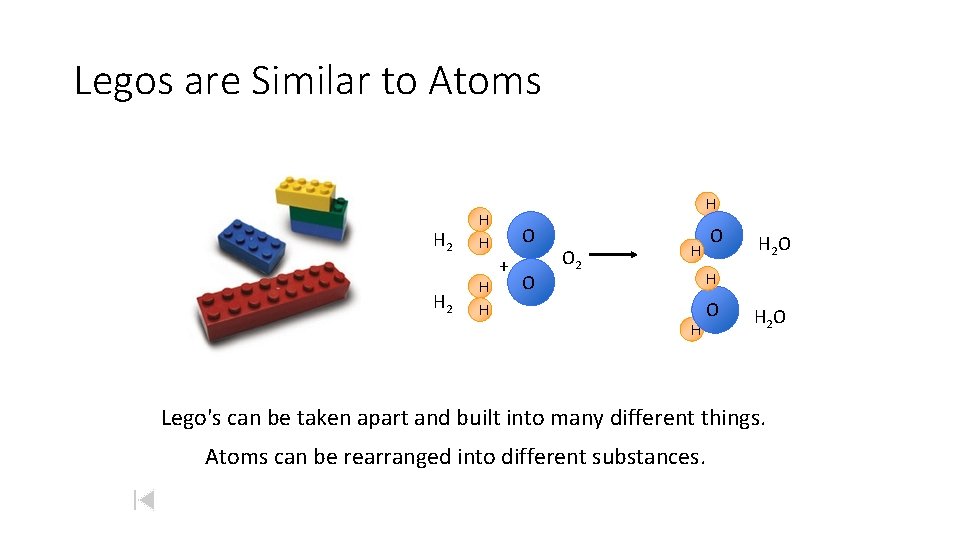
Legos are Similar to Atoms H 2 H H H O + O O 2 H O H 2 O H H O H 2 O Lego's can be taken apart and built into many different things. Atoms can be rearranged into different substances.

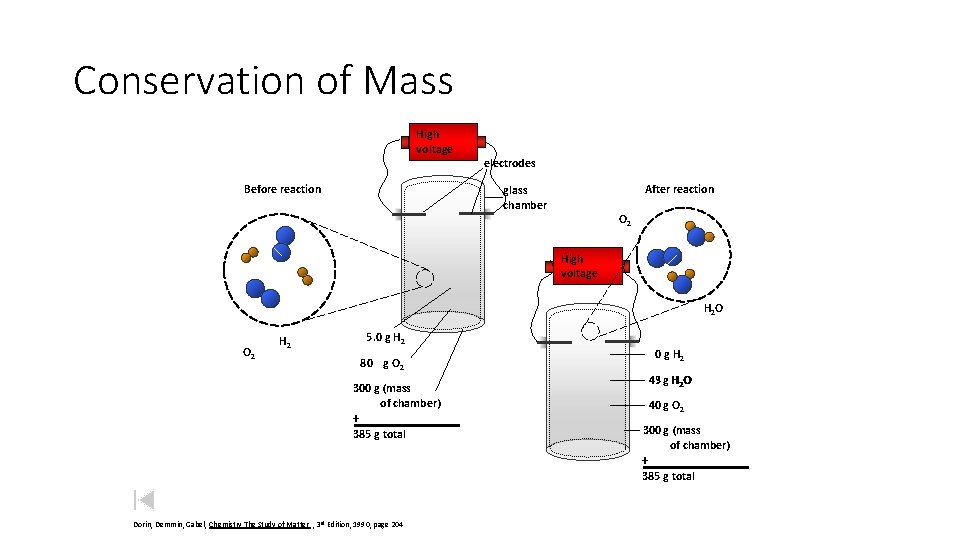
Conservation of Mass High voltage Before reaction electrodes After reaction glass chamber O 2 High voltage H 2 O O 2 5. 0 g H 2 80 g O 2 45 ? g H 2 O 300 g (mass of chamber) + 385 g total 40 g O 2 300 g (mass of chamber) + 385 g total Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 204

Law of Definite Proportions Joseph Louis Proust (1754 – 1826) • Each compound has a specific ratio of elements • It is a ratio by mass • Water is always 8 grams of oxygen for every one gram of hydrogen

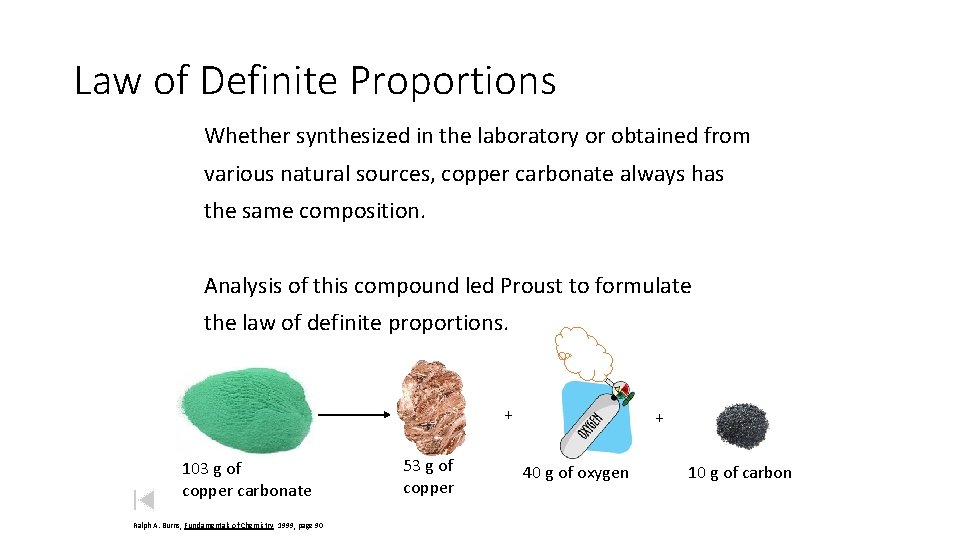
Law of Definite Proportions Whether synthesized in the laboratory or obtained from various natural sources, copper carbonate always has the same composition. Analysis of this compound led Proust to formulate the law of definite proportions. + 103 g of copper carbonate Ralph A. Burns, Fundamentals of Chemistry 1999, page 90 53 g of copper + 40 g of oxygen 10 g of carbon

What? • Water is 8 grams of oxygen per gram of hydrogen. • Hydrogen peroxide is 16 grams of oxygen per gram of hydrogen. • 16 g to 8 g is a 2: 1 ratio • True, because you have to add a whole atom, you can’t add a piece of an atom.

Daltons Atomic Theory • Dalton stated that elements consisted of tiny particles called atoms • He also called the elements pure substances because all atoms of an element were identical and that in particular they had the same mass.

Dalton’s Atomic Theory. 1. All matter is made of tiny indivisible particles called atoms. “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. 4. All atoms of the same element are identical in mass, size, and other properties. 5. Atoms of one element differ in mass and other properties from atoms of other elements. 6. In compounds, atoms of different elements combine in simple, whole number ratios.

The Atomic Theory of Matter • Dalton’s atomic theory is essentially correct, with four minor modifications: 1. Not all atoms of an element must have precisely the same mass. 2. Atoms of one element can be transformed into another through nuclear reactions. 3. The composition of many solid compounds are somewhat 4. Under certain circumstances, some atoms can be divided (split into smaller particles: i. e. nuclear fission). Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. variable.

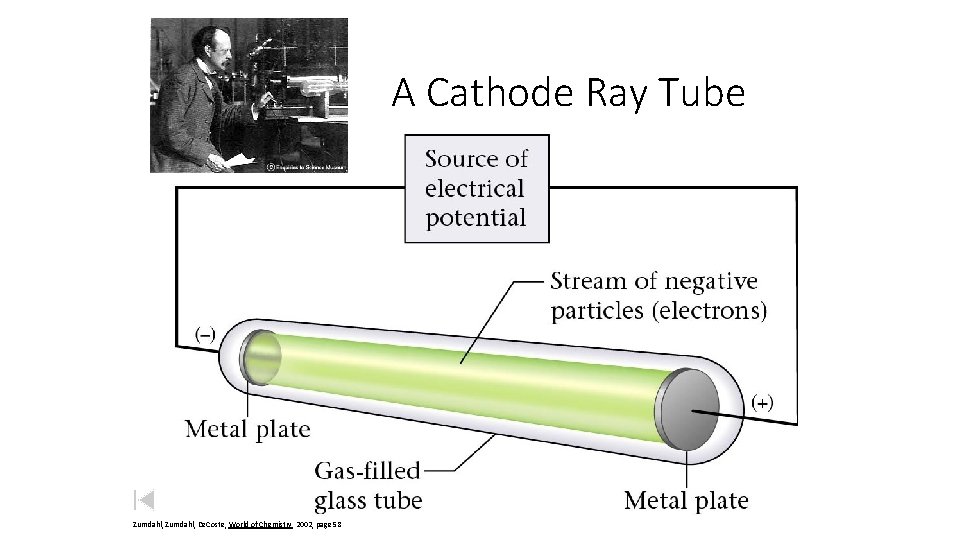
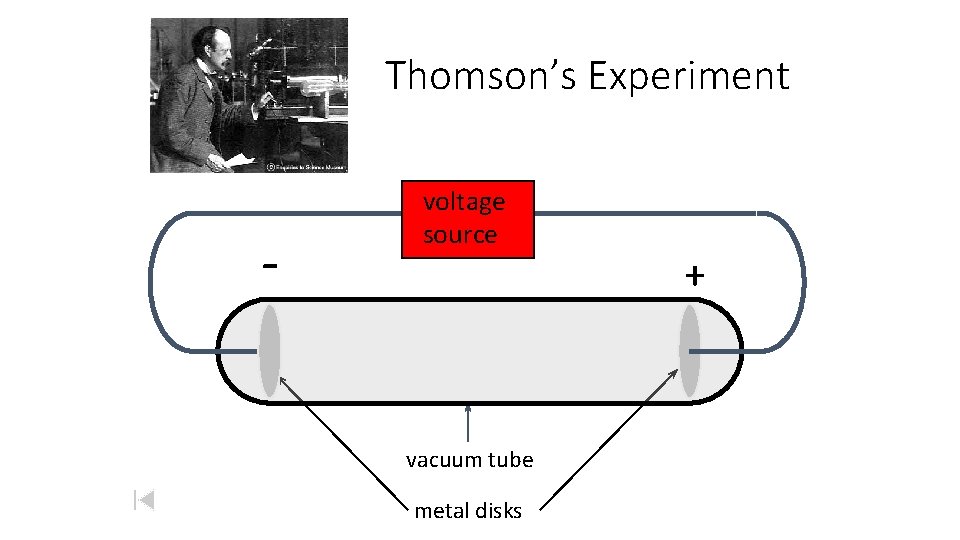
Thomson Model of the Atom J. J. Thomson - English physicist. 1897 Made a piece of equipment called a cathode ray tube. It is a vacuum tube - all the air has been pumped out.

A Cathode Ray Tube Zumdahl, De. Coste, World of Chemistry 2002, page 58

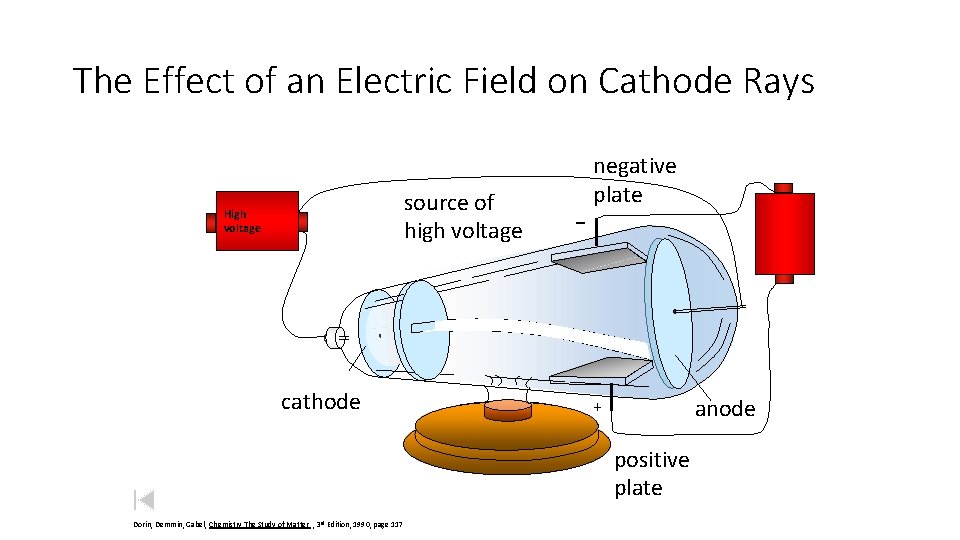
The Effect of an Electric Field on Cathode Rays source of high voltage High voltage cathode _ negative plate anode + positive plate Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 117

Thomson’s Experiment - voltage source vacuum tube metal disks +

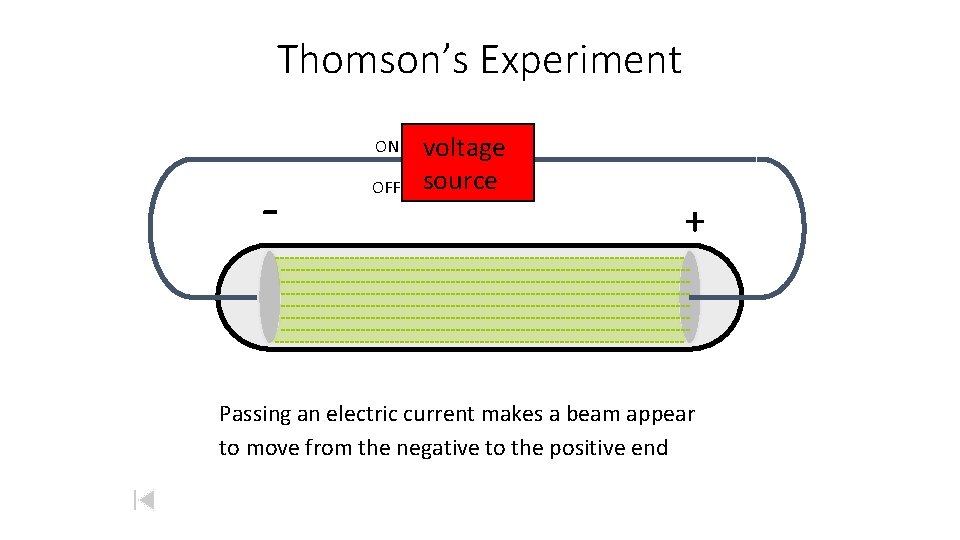
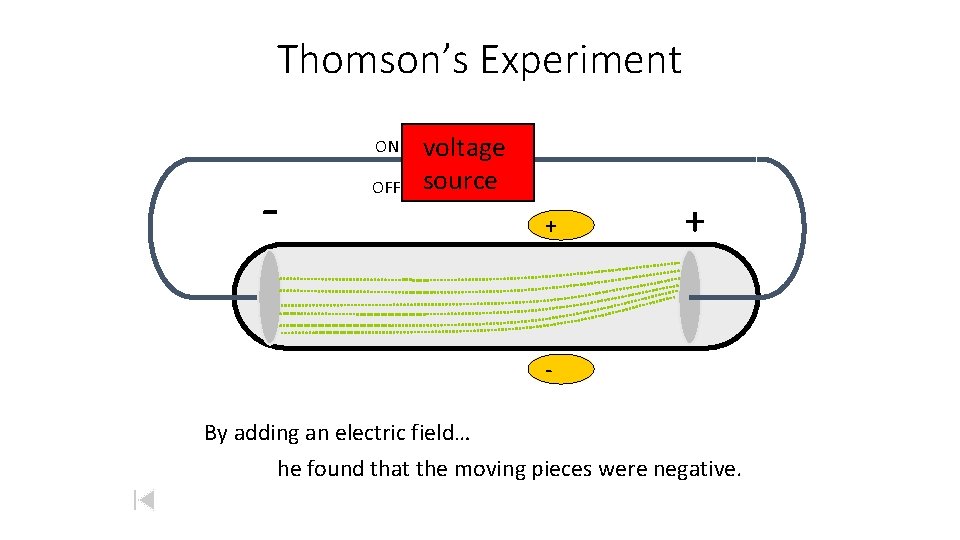
Thomson’s Experiment ON - OFF voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end

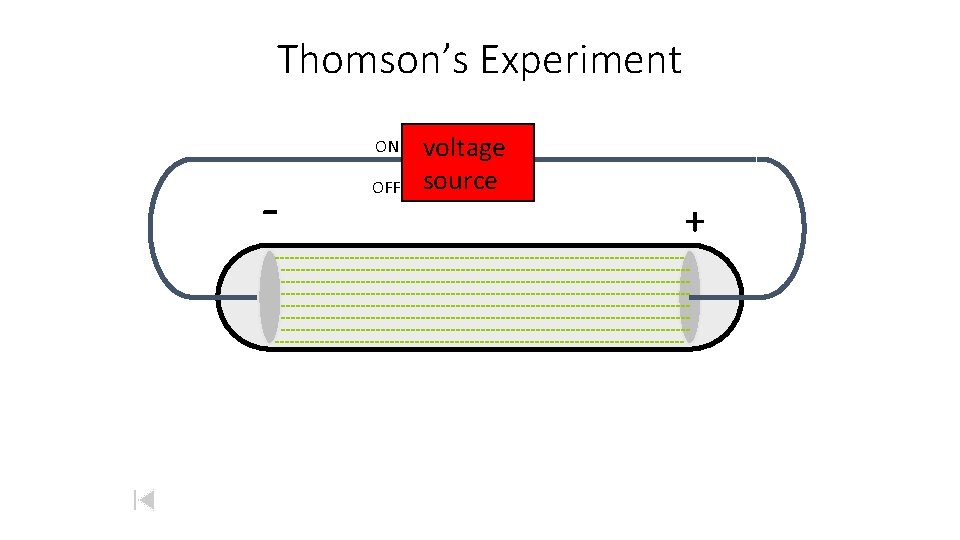
Thomson’s Experiment ON - OFF voltage source +

Thomson’s Experiment ON - OFF voltage source + + By adding an electric field… he found that the moving pieces were negative.

Video 1 Video 2

J. J. Thomson • He proved that atoms of any element can be made to emit tiny negative particles. • He conducted the experiment with different gases and got the same result • From this he concluded that ALL atoms must contain these negative particles. • He named these particles ELECTRONS.

Thomson Model of the Atom • J. J. Thomson discovered the electron and knew that electrons could be emitted from matter (1897) • He explained that the electrons are li • He knew that atoms did not have a net negative charge and so there must be balancing the negative charge.

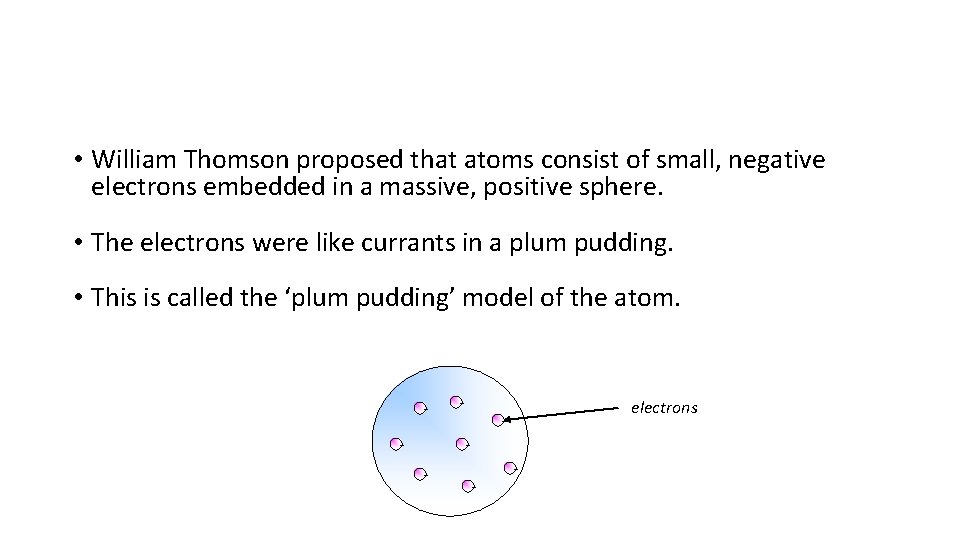
• William Thomson proposed that atoms consist of small, negative electrons embedded in a massive, positive sphere. • The electrons were like currants in a plum pudding. • This is called the ‘plum pudding’ model of the atom. - - electrons - -

Goldstein - 1886 • Used modified type of discharge tube with a perforated cathode

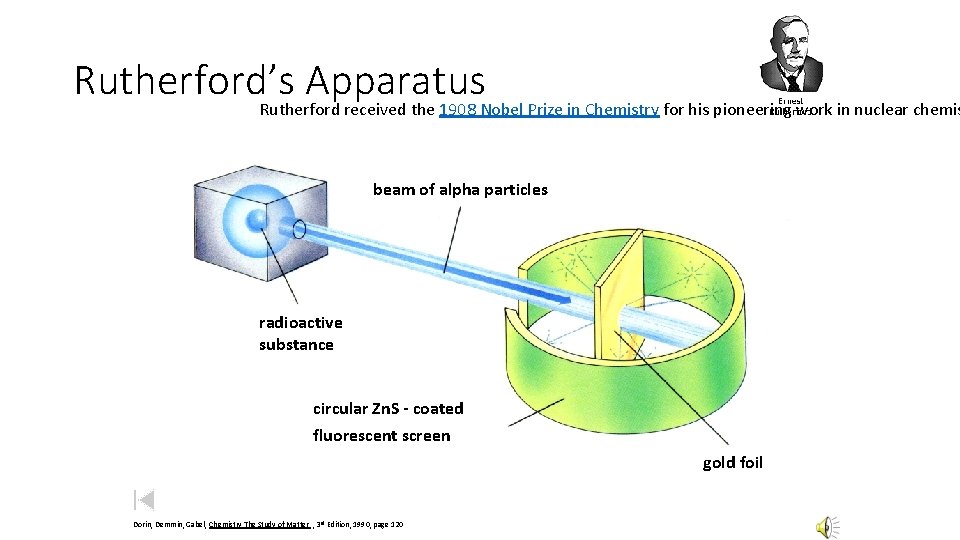
Rutherford’s Apparatus Rutherford received the 1908 Nobel Prize in Chemistry for his pioneering work in nuclear chemis beam of alpha particles radioactive substance circular Zn. S - coated fluorescent screen gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 120

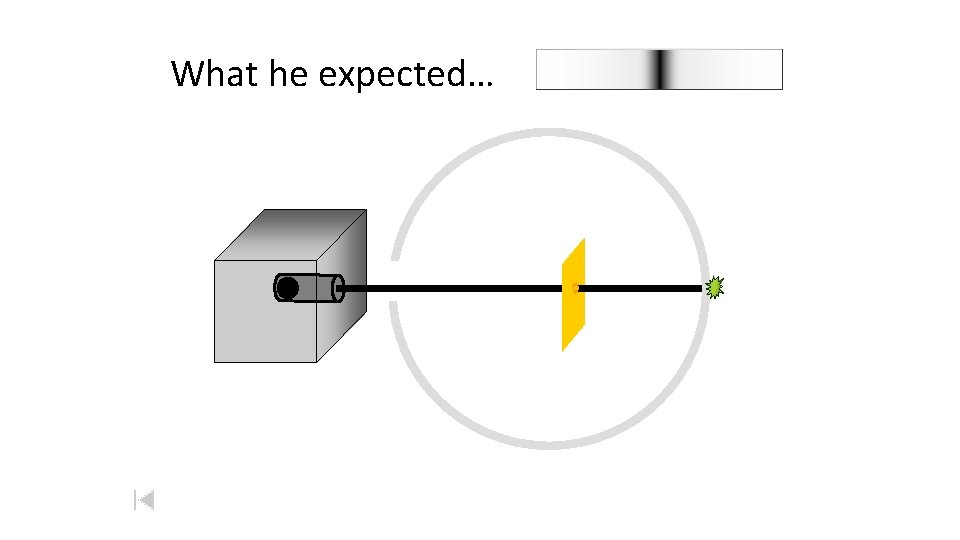
What he expected…

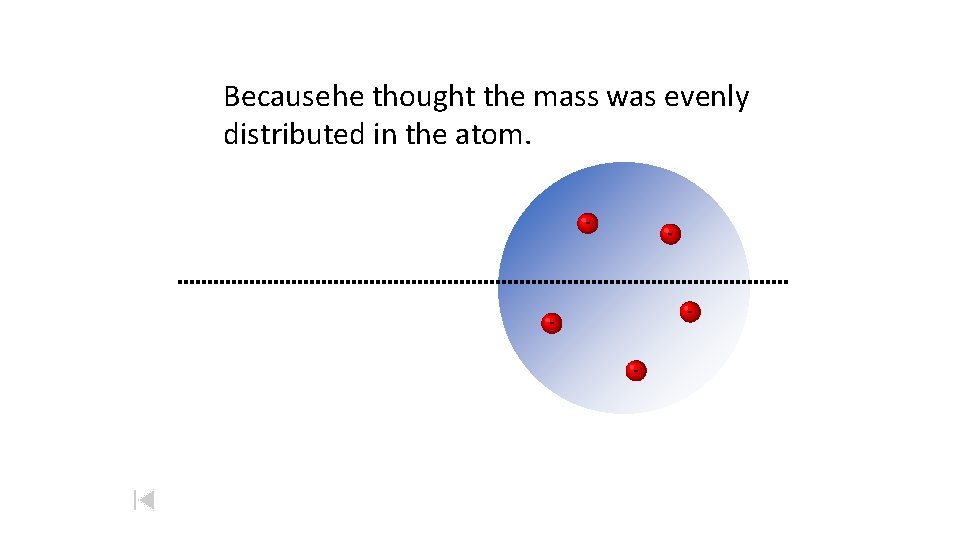
Because he thought the mass was evenly distributed in the atom. - -

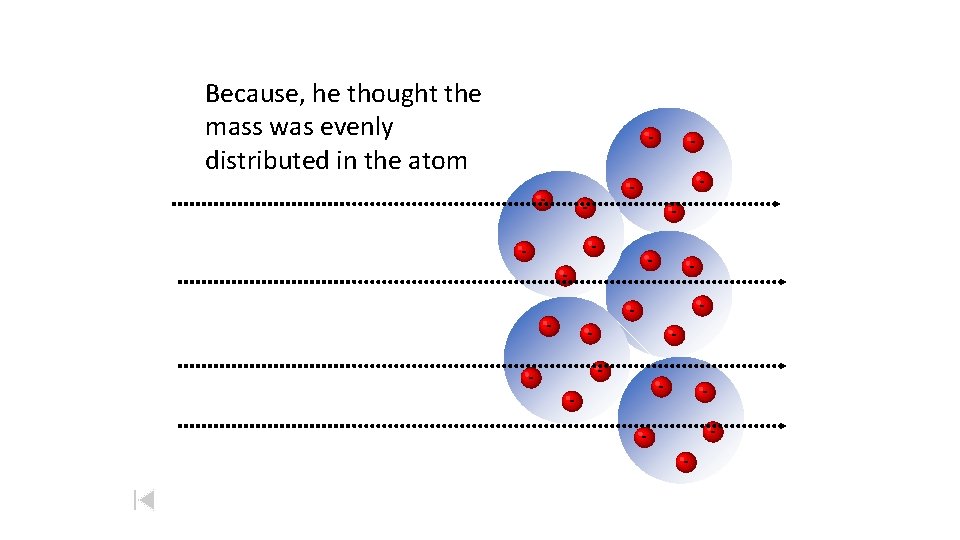
Because, he thought the mass was evenly distributed in the atom --- -- --- -- -- ----

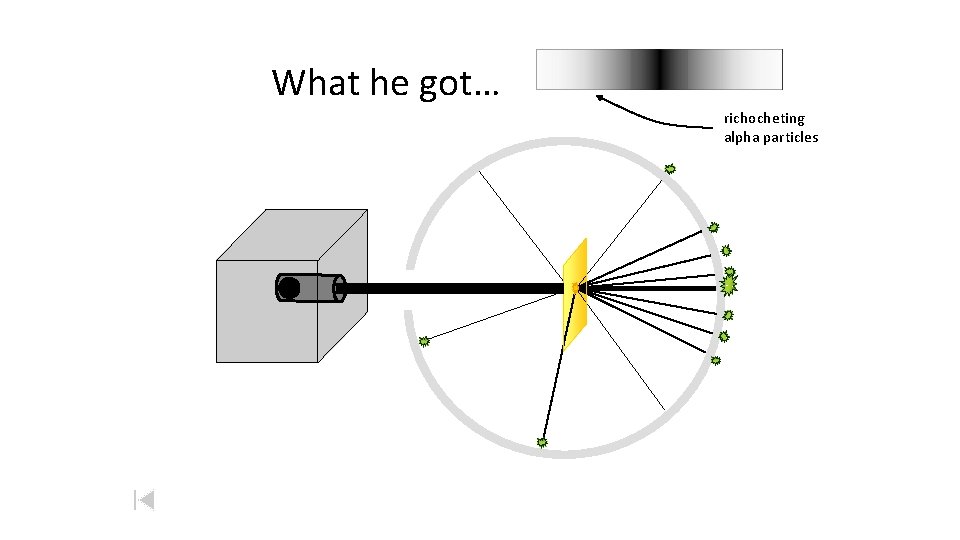
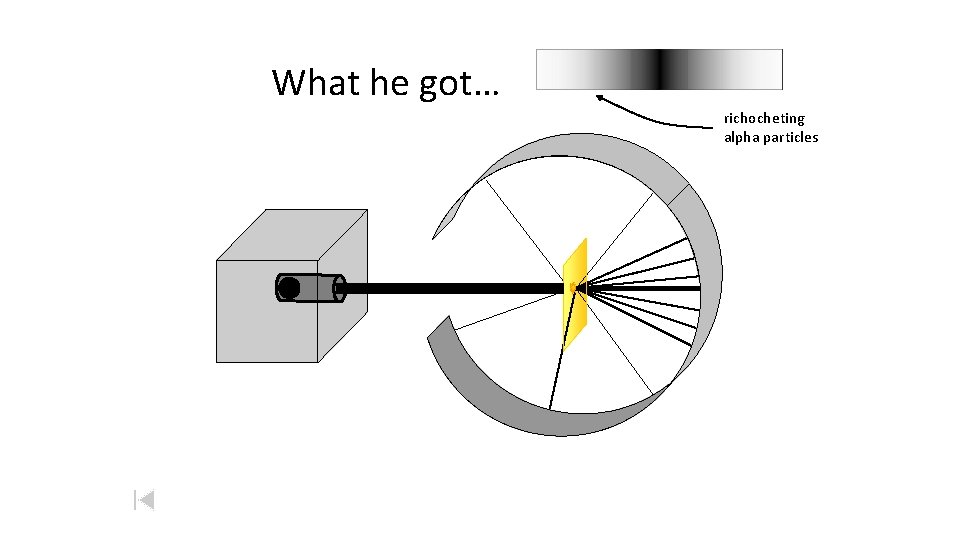
What he got… richocheting alpha particles

What he got… richocheting alpha particles

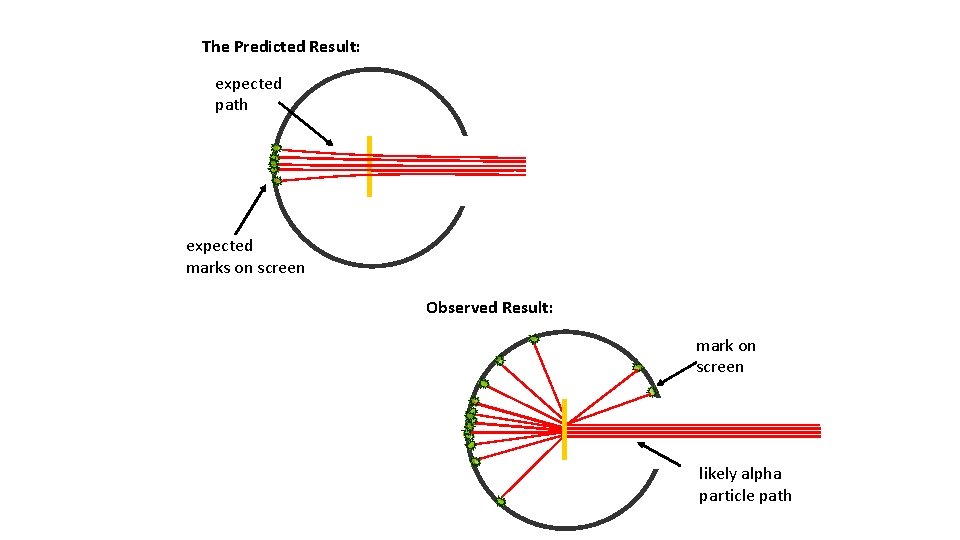
The Predicted Result: expected path expected marks on screen Observed Result: mark on screen likely alpha particle path

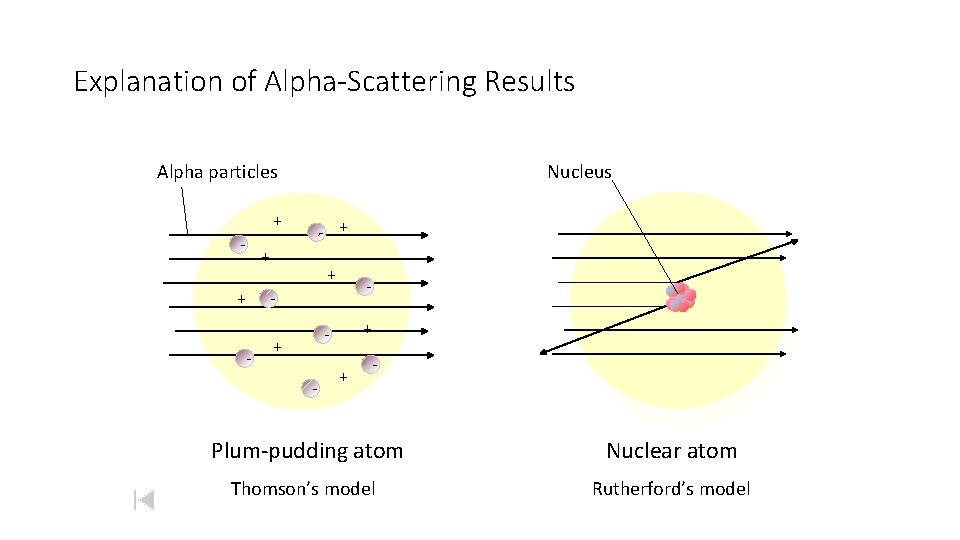
Explanation of Alpha-Scattering Results Alpha particles Nucleus + - - + + + - - + - + - Plum-pudding atom Nuclear atom Thomson’s model Rutherford’s model

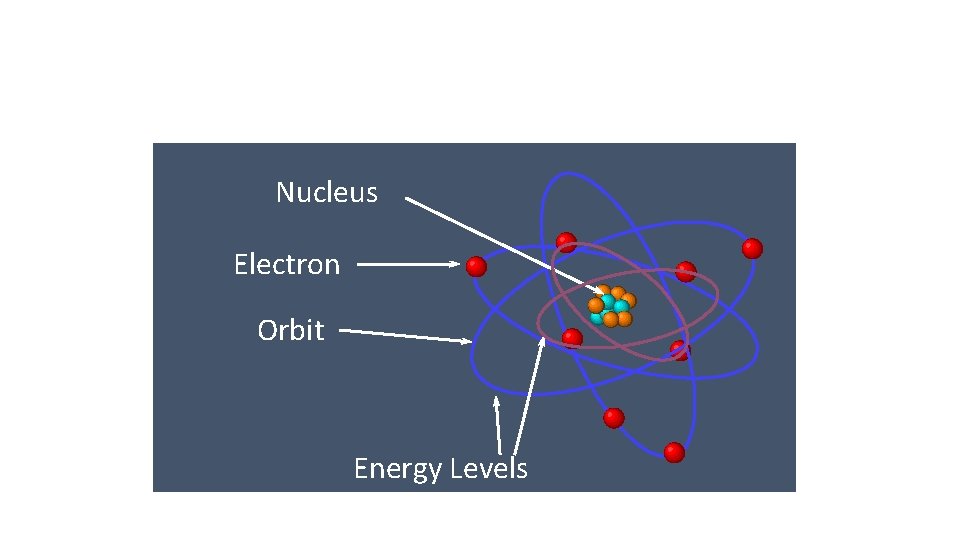
Bohr’s Model Nucleus Electron Orbit Energy Levels

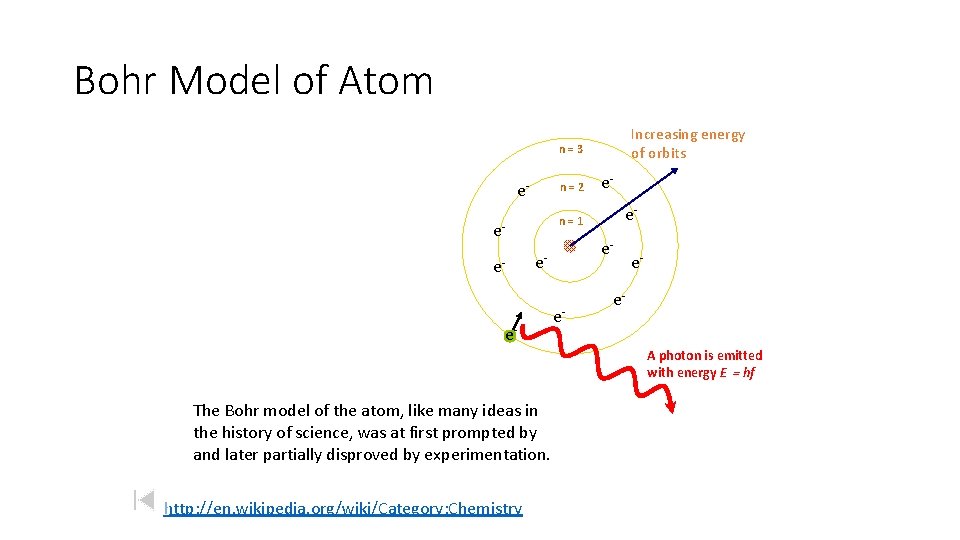
Bohr Model of Atom Increasing energy of orbits n=3 e- n=2 e- n=1 ee- e- e- e. A photon is emitted with energy E = hf The Bohr model of the atom, like many ideas in the history of science, was at first prompted by and later partially disproved by experimentation. http: //en. wikipedia. org/wiki/Category: Chemistry

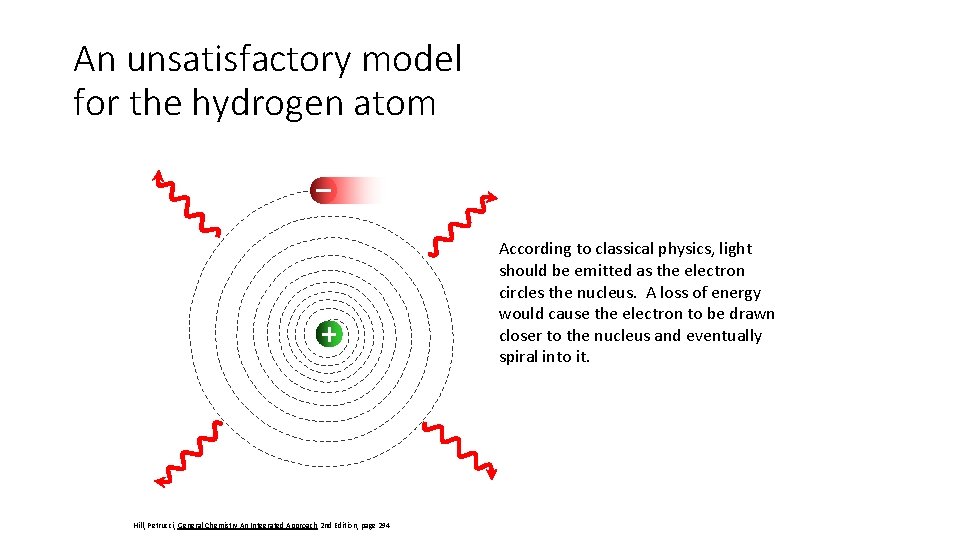
An unsatisfactory model for the hydrogen atom According to classical physics, light should be emitted as the electron circles the nucleus. A loss of energy would cause the electron to be drawn closer to the nucleus and eventually spiral into it. Hill, Petrucci, General Chemistry An Integrated Approach 2 nd Edition, page 294