Development of reference panels for serological testing Intended






- Slides: 29

Development of reference panels for serological testing Intended use, fitness for purpose Amadeo Sáez-Alquézar Second WHO Consultation: Development of a WHO reference panel for the control of Chagas diagnostic tests Geneva - 2009

Serological Screening and Diagnostic of infectious diseases Quality Control Procedures Serum Panels as a Reference An indispensable tool

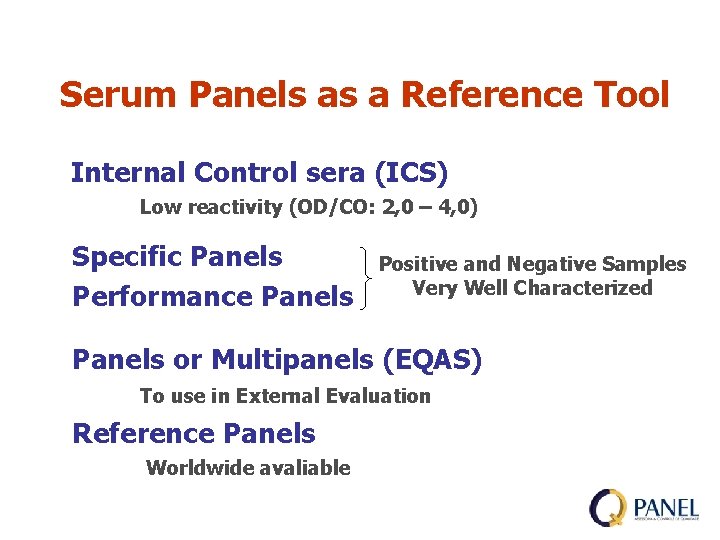
Serum Panels as a Reference Tool Internal Control sera (ICS) Low reactivity (OD/CO: 2, 0 – 4, 0) Specific Panels Performance Panels Positive and Negative Samples Very Well Characterized Panels or Multipanels (EQAS) To use in External Evaluation Reference Panels Worldwide avaliable

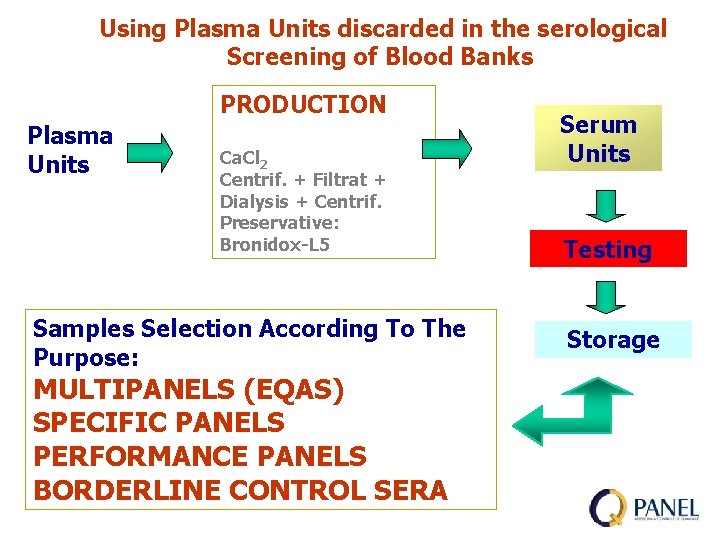
Using Plasma Units discarded in the serological Screening of Blood Banks PRODUCTION Plasma Units Ca. Cl 2 Centrif. + Filtrat + Dialysis + Centrif. Preservative: Bronidox-L 5 Samples Selection According To The Purpose: MULTIPANELS (EQAS) SPECIFIC PANELS PERFORMANCE PANELS BORDERLINE CONTROL SERA Serum Units Testing Storage

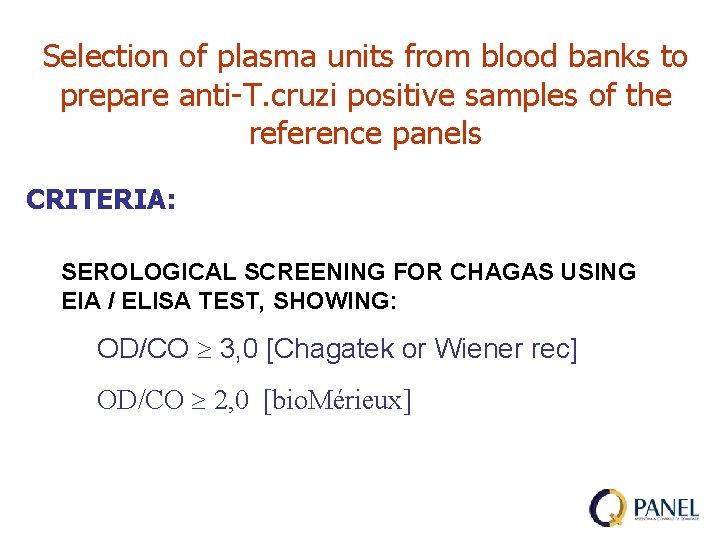
Selection of plasma units from blood banks to prepare anti-T. cruzi positive samples of the reference panels CRITERIA: SEROLOGICAL SCREENING FOR CHAGAS USING EIA / ELISA TEST, SHOWING: OD/CO 3, 0 [Chagatek or Wiener rec] OD/CO 2, 0 [bio. Mérieux]

Testing Sera Obtained from Plasma Units Several ELISA Tests (Lys and rec) 1 – 3 IHA Tests

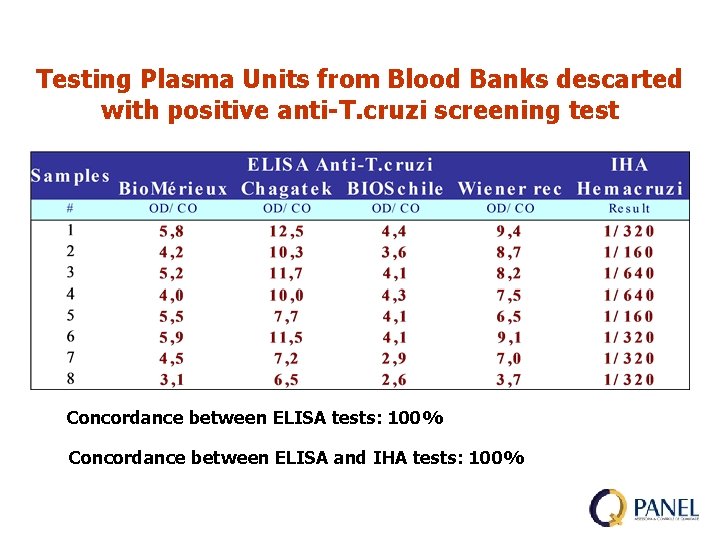
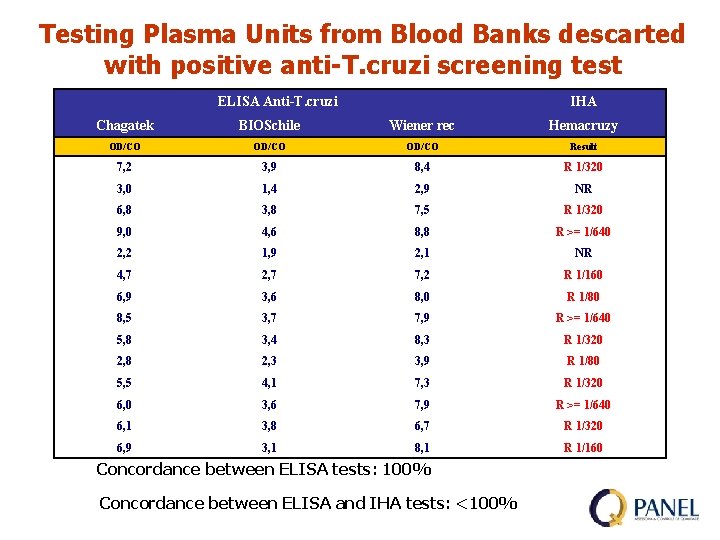
Testing Plasma Units from Blood Banks descarted with positive anti-T. cruzi screening test Concordance between ELISA tests: 100% Concordance between ELISA and IHA tests: 100%

Testing Plasma Units from Blood Banks descarted with positive anti-T. cruzi screening test ELISA Anti-T. cruzi IHA Chagatek BIOSchile Wiener rec Hemacruzy OD/CO Result 7, 2 3, 9 8, 4 R 1/320 3, 0 1, 4 2, 9 NR 6, 8 3, 8 7, 5 R 1/320 9, 0 4, 6 8, 8 R >= 1/640 2, 2 1, 9 2, 1 NR 4, 7 2, 7 7, 2 R 1/160 6, 9 3, 6 8, 0 R 1/80 8, 5 3, 7 7, 9 R >= 1/640 5, 8 3, 4 8, 3 R 1/320 2, 8 2, 3 3, 9 R 1/80 5, 5 4, 1 7, 3 R 1/320 6, 0 3, 6 7, 9 R >= 1/640 6, 1 3, 8 6, 7 R 1/320 6, 9 3, 1 8, 1 R 1/160 Concordance between ELISA tests: 100% Concordance between ELISA and IHA tests: <100%

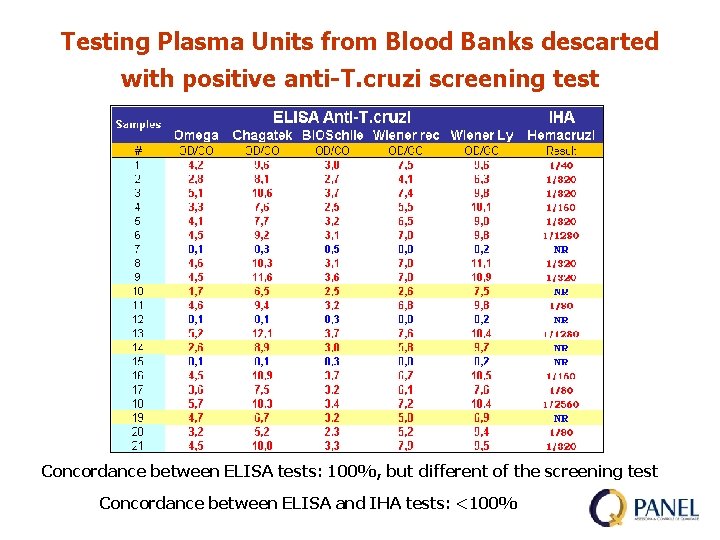
Testing Plasma Units from Blood Banks descarted with positive anti-T. cruzi screening test Concordance between ELISA tests: 100%, but different of the screening test Concordance between ELISA and IHA tests: <100%

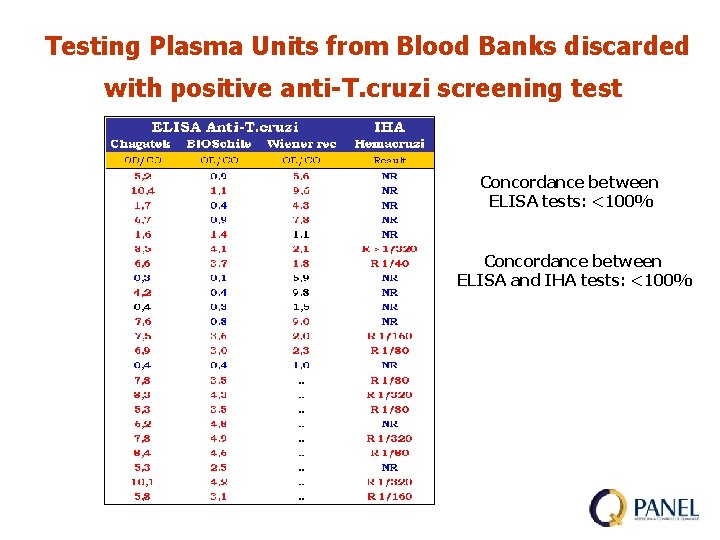
Testing Plasma Units from Blood Banks discarded with positive anti-T. cruzi screening test Concordance between ELISA tests: <100% Concordance between ELISA and IHA tests: <100%

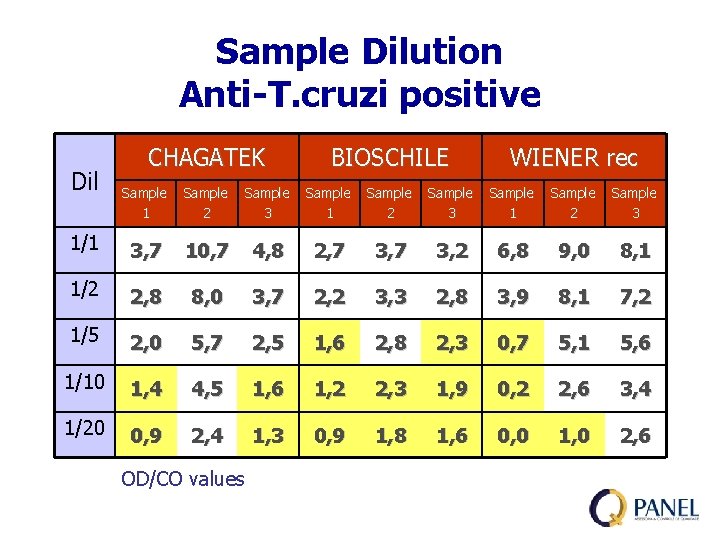
Sample Dilution To prepare sera panels must be necessary obtain adequate volume of each sample. For this purpose should be necessary dilute samples, with negative serum or by mixture with other positive samples. It is important to observe some criteria to make dilutions

Sample Dilution Anti-T. cruzi positive Dil CHAGATEK BIOSCHILE WIENER rec Sample 1 Sample 2 Sample 3 1/1 3, 7 10, 7 4, 8 2, 7 3, 2 6, 8 9, 0 8, 1 1/2 2, 8 8, 0 3, 7 2, 2 3, 3 2, 8 3, 9 8, 1 7, 2 1/5 2, 0 5, 7 2, 5 1, 6 2, 8 2, 3 0, 7 5, 1 5, 6 1/10 1, 4 4, 5 1, 6 1, 2 2, 3 1, 9 0, 2 2, 6 3, 4 1/20 0, 9 2, 4 1, 3 0, 9 1, 8 1, 6 0, 0 1, 0 2, 6 OD/CO values

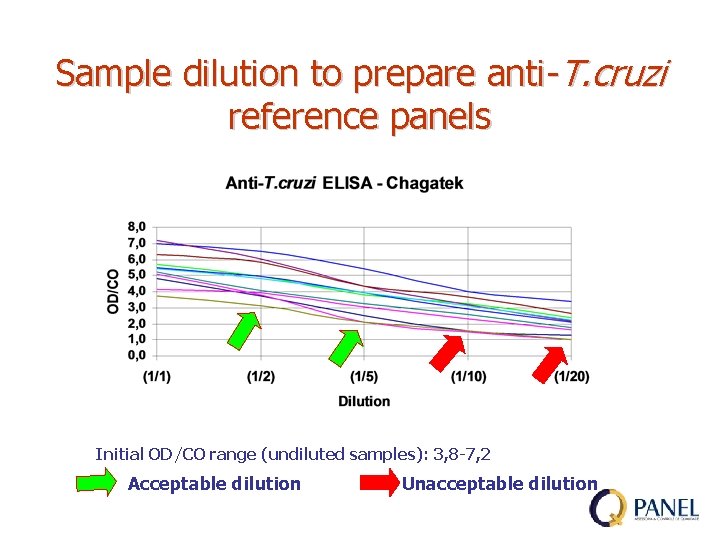
Sample dilution to prepare anti-T. cruzi reference panels Initial OD/CO range (undiluted samples): 3, 8 -7, 2 Acceptable dilution Unacceptable dilution

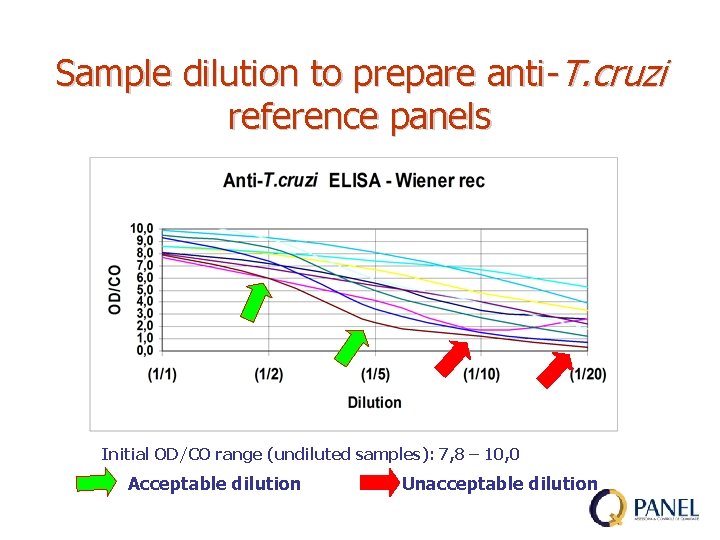
Sample dilution to prepare anti-T. cruzi reference panels Initial OD/CO range (undiluted samples): 7, 8 – 10, 0 Acceptable dilution Unacceptable dilution

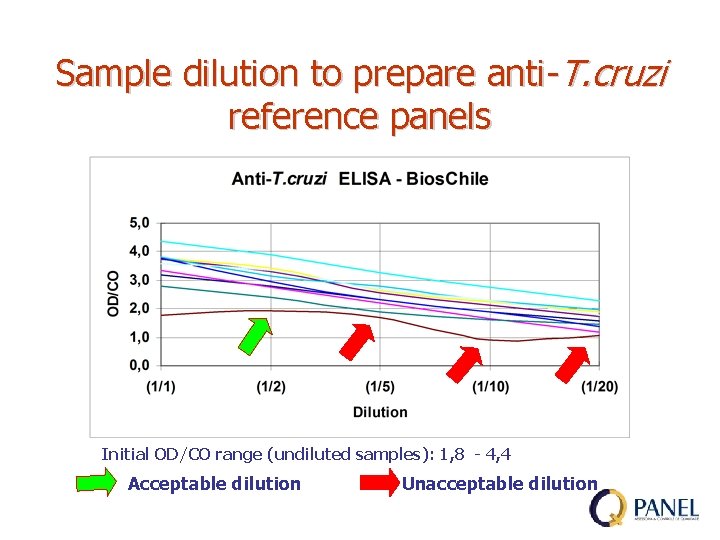
Sample dilution to prepare anti-T. cruzi reference panels Initial OD/CO range (undiluted samples): 1, 8 - 4, 4 Acceptable dilution Unacceptable dilution

Samples Characterization • We consider the more important step to assess the quality of panels

Testing samples ü Tests used in the serological screening are qualitative tests • determines whether the substance being tested for is present or absent ü Results obtained by the PL will be compared with a reference panel sent by de Organizer Center. So the reference panel must be very well tested, to assure the certainty of the results

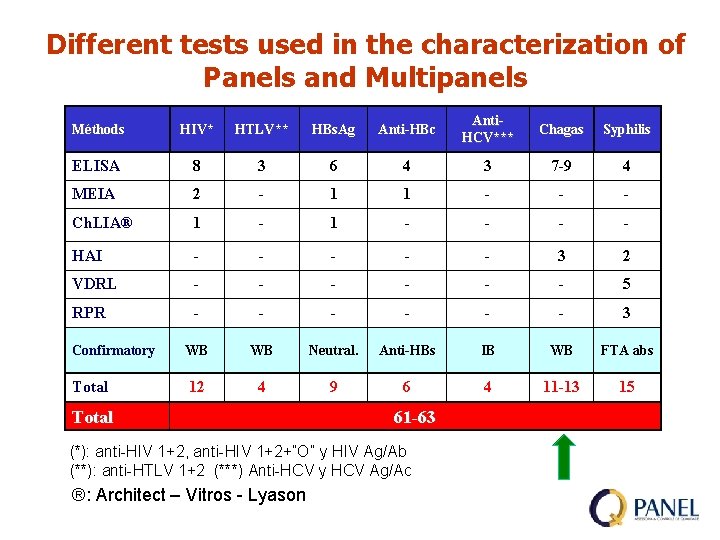
Different tests used in the characterization of Panels and Multipanels Méthods HIV* HTLV** HBs. Ag Anti-HBc Anti. HCV*** Chagas Syphilis ELISA 8 3 6 4 3 7 -9 4 MEIA 2 - 1 1 - - - Ch. LIA® 1 - - - - HAI - - - 3 2 VDRL - - - 5 RPR - - - 3 Confirmatory WB WB Neutral. Anti-HBs IB WB FTA abs Total 12 4 9 6 4 11 -13 15 Total 61 -63 (*): anti-HIV 1+2, anti-HIV 1+2+”O” y HIV Ag/Ab (**): anti-HTLV 1+2 (***) Anti-HCV y HCV Ag/Ac ®: Architect – Vitros - Lyason

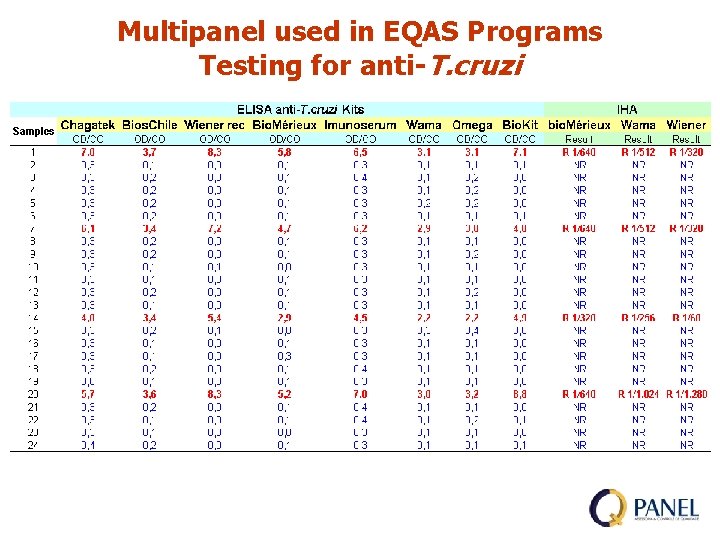
Multipanel used in EQAS Programs Testing for anti-T. cruzi

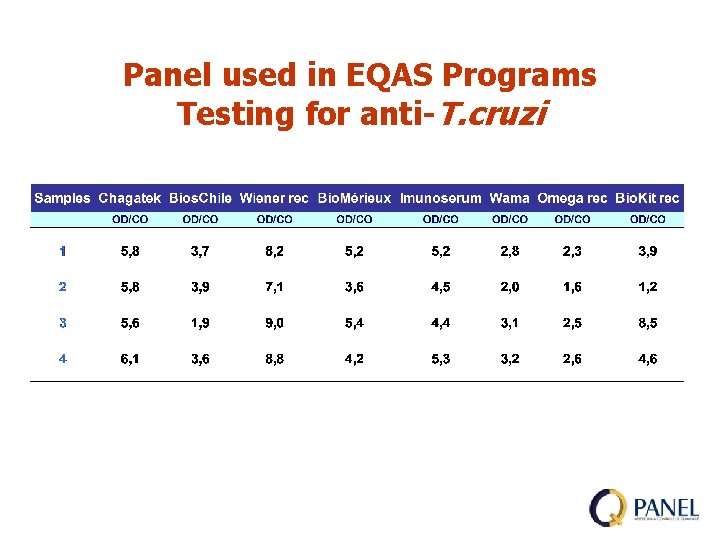
Panel used in EQAS Programs Testing for anti-T. cruzi

External Quality Assessment • Blind Panels üFor a single screening test (f. instance: anti-T. cruzi) üN = 5 to 10 samples 5 -7 positive and 3 -5 negative üTesting For at least 6 different ELISA tests Two IHA tests If possible, one complemmentary test

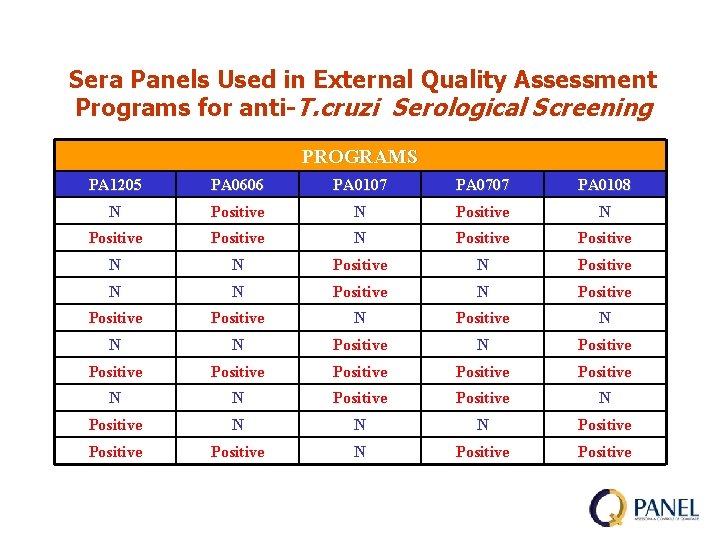
Sera Panels Used in External Quality Assessment Programs for anti-T. cruzi Serological Screening PROGRAMS PA 1205 PA 0606 PA 0107 PA 0707 PA 0108 N Positive N Positive Positive N Positive N N N Positive Positive N N Positive N N N Positive N Positive

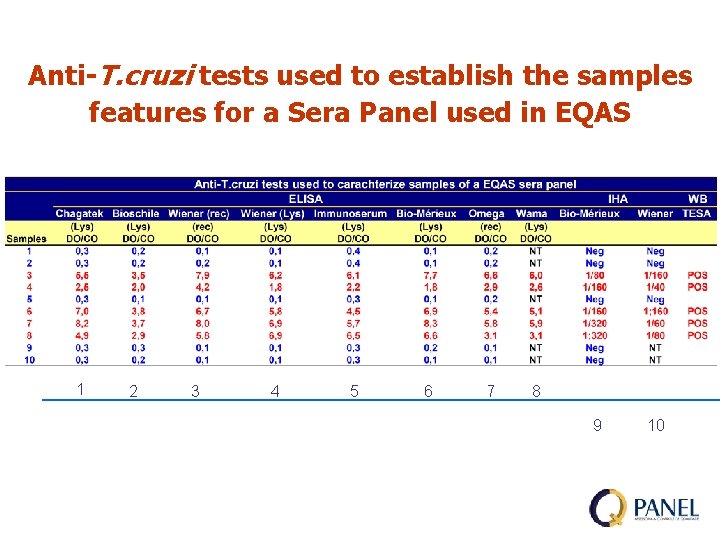
Anti-T. cruzi tests used to establish the samples features for a Sera Panel used in EQAS 1 2 3 4 5 6 7 8 9 10

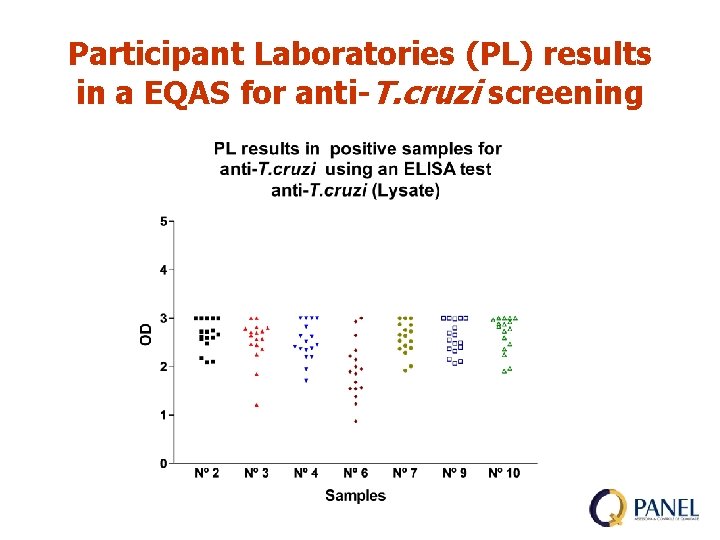
Participant Laboratories (PL) results in a EQAS for anti-T. cruzi screening

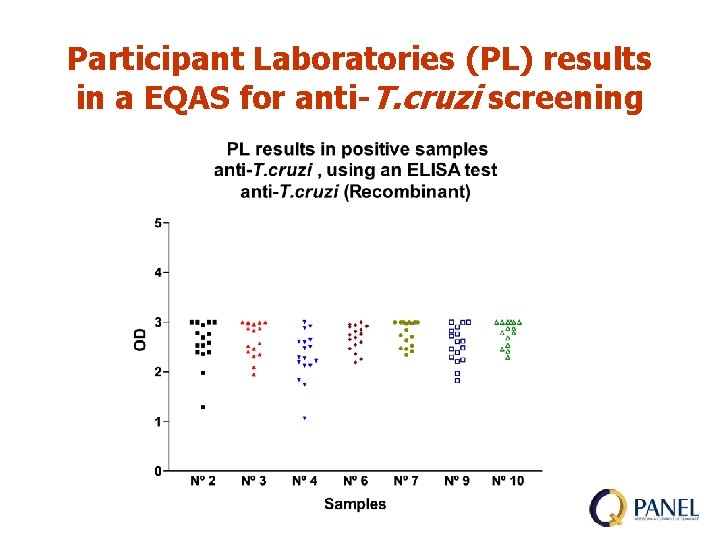
Participant Laboratories (PL) results in a EQAS for anti-T. cruzi screening

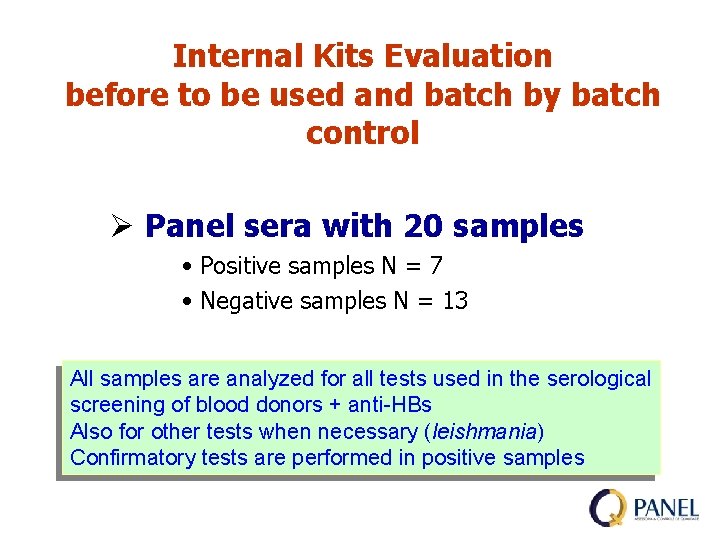
Internal Kits Evaluation before to be used and batch by batch control Ø Panel sera with 20 samples • Positive samples N = 7 • Negative samples N = 13 All samples are analyzed for all tests used in the serological screening of blood donors + anti-HBs Also for other tests when necessary (leishmania) Confirmatory tests are performed in positive samples

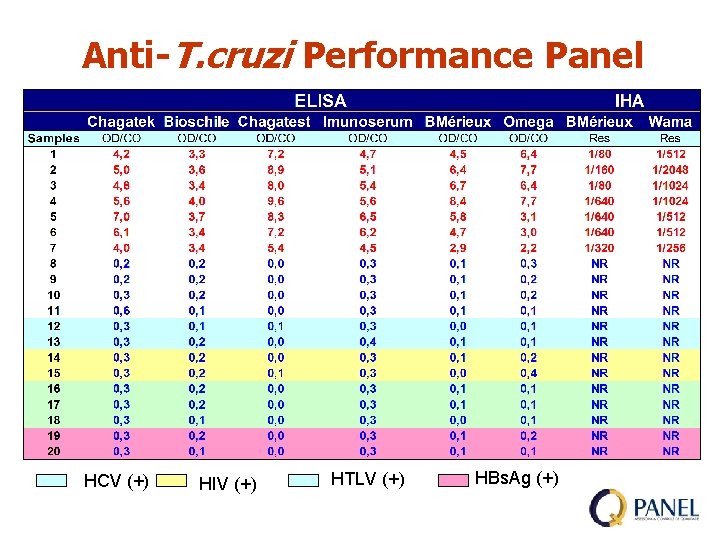
Anti-T. cruzi Performance Panel HCV (+) HIV (+) HTLV (+) HBs. Ag (+)

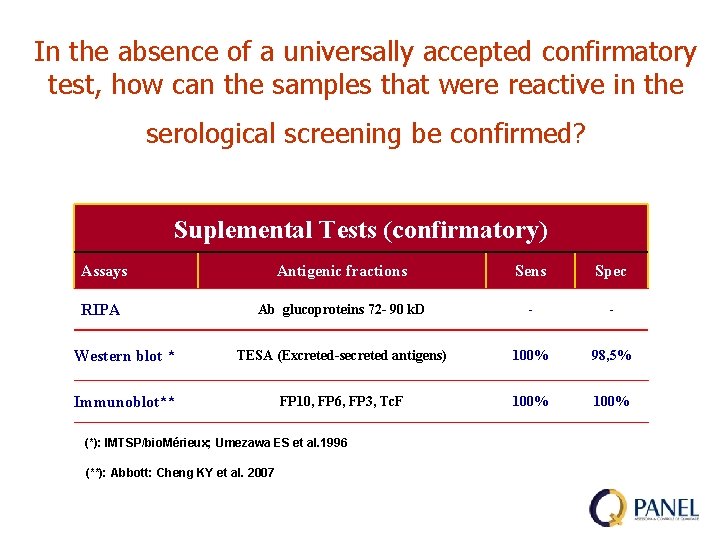
In the absence of a universally accepted confirmatory test, how can the samples that were reactive in the serological screening be confirmed? Suplemental Tests (confirmatory) Assays Antigenic fractions Sens Spec RIPA Ab glucoproteins 72 - 90 k. D - - Western blot * TESA (Excreted-secreted antigens) 100% 98, 5% Immunoblot** FP 10, FP 6, FP 3, Tc. F 100% (*): IMTSP/bio. Mérieux; Umezawa ES et al. 1996 (**): Abbott: Cheng KY et al. 2007

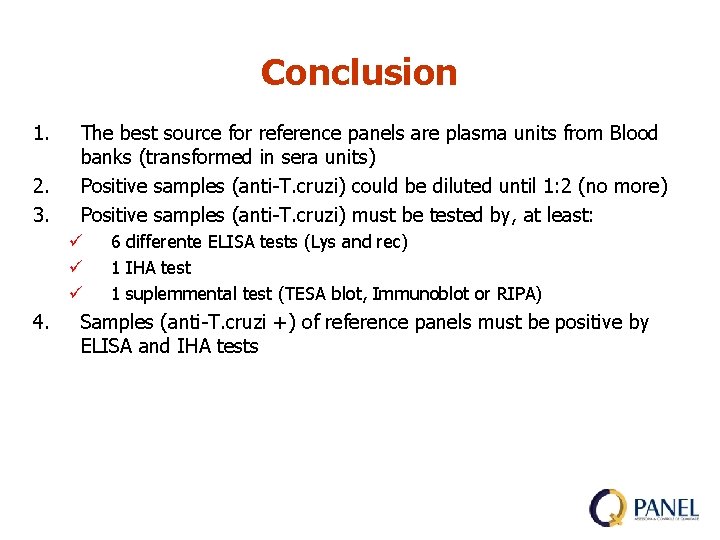
Conclusion 1. 2. 3. The best source for reference panels are plasma units from Blood banks (transformed in sera units) Positive samples (anti-T. cruzi) could be diluted until 1: 2 (no more) Positive samples (anti-T. cruzi) must be tested by, at least: ü ü ü 4. 6 differente ELISA tests (Lys and rec) 1 IHA test 1 suplemmental test (TESA blot, Immunoblot or RIPA) Samples (anti-T. cruzi +) of reference panels must be positive by ELISA and IHA tests