Development of Pristina TM Aerogel for Targeted and


![TAASI Project Goals 1. Develop very fine particles [less than 2 microns] with 90% TAASI Project Goals 1. Develop very fine particles [less than 2 microns] with 90%](https://slidetodoc.com/presentation_image/928592acef00bc5d36df5c6770853937/image-3.jpg)



- Slides: 11

Development of Pristina. TM Aerogel for Targeted and Controlled Release of Cancer Pharmaceuticals The ATTIA Applied Sciences Inc. TAASI Corporation Page 1

Schematic of Therapeutic Nanodevice “Stealth” Coating Agent Aerogel Particle Agent Releasing Aerogel Particle Near Cell Membrane Receptor on cell surface Ligand Receptor Target Cell Particle device binds to target cell. A) Page 2 Cell Wall Target Cell Therapeutic agent is released from pore structure of device. B)
![TAASI Project Goals 1 Develop very fine particles less than 2 microns with 90 TAASI Project Goals 1. Develop very fine particles [less than 2 microns] with 90%](https://slidetodoc.com/presentation_image/928592acef00bc5d36df5c6770853937/image-3.jpg)
TAASI Project Goals 1. Develop very fine particles [less than 2 microns] with 90% porosity of TAASI’s Pristina. TM SCM-Aerogel for the storage and subsequent 60 minute controlled release of targeted pharmaceutical agents (melitin). 2. Integrate the chemical “o, o-Bis{3 aminopropyl) polyethylene glycol”, which acts as the “stealth” & anchoring link to the tumor specific EGF (Epidermal Growth Factor Ligand) protein. 3. Gadolinium Oxide, Gd 2 O 3 [gadolinia], to be incorporated into the aerogel to act as a detection tag/identification label. Page 3

Evaluation Procedures: Aerogel Effectiveness for Targeted/Controlled Release Pharmaceuticals Routine experiments were outsourced to determine the effectiveness of the TAASI Aerogel particles in the melitin delivery system to targeted Cancer cells. § Aerogel Particle Preparatory Methods o § Model of Cancer Cells: Swiss 3 T 3 Cells o § Coupled with EGF (Epidermal Growth Factor Ligand) and loaded with melitin prior to “seek and destroy” experiment Expresses high levels of EGF Receptors Model of Non-Cancer Cells: CHO Cells Page 4 o Cell concentration was adjusted to equal the Swiss 3 T 3 o No EGF Receptors Present on CHO cells

TAASI Microgel SCM-PEG-Gd Aerogel Page 5

Size Reduction Methods 1. Micronization, by Sonication, was used to achieve the desired aerogel particle size of 1 to 2 microns. 1. Fractionation, by gravity settling, was use to separate out the desired aerogel particles. Page 6

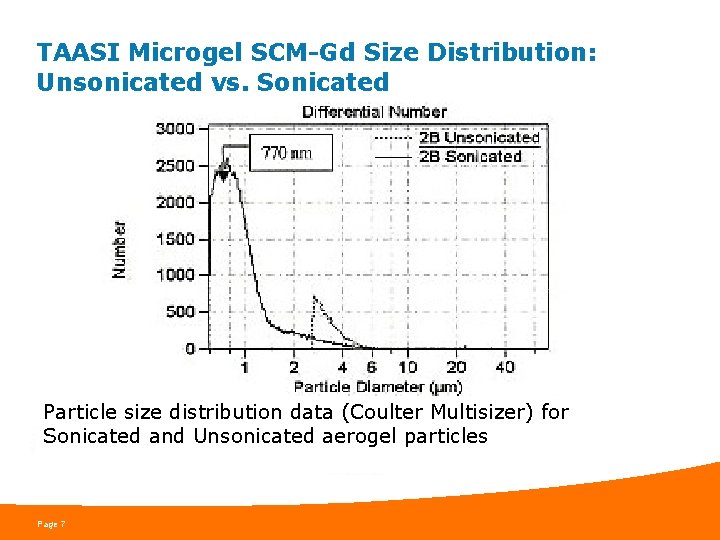
TAASI Microgel SCM-Gd Size Distribution: Unsonicated vs. Sonicated Particle size distribution data (Coulter Multisizer) for Sonicated and Unsonicated aerogel particles Page 7

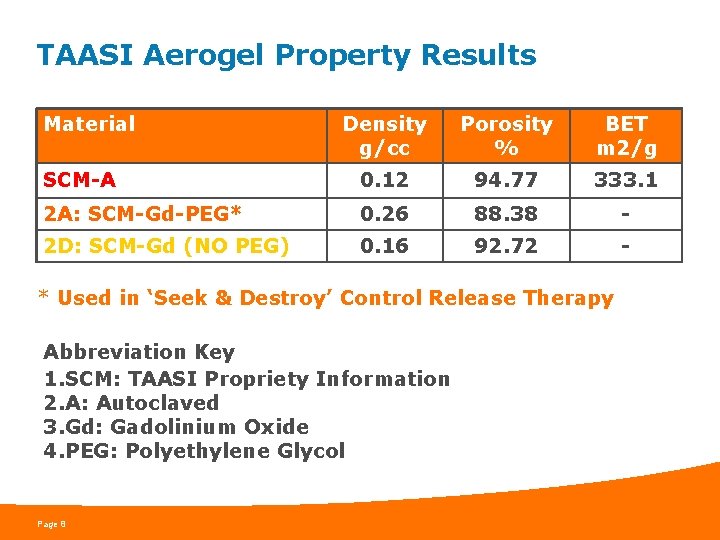
TAASI Aerogel Property Results Material Density g/cc Porosity % BET m 2/g SCM-A 0. 12 94. 77 333. 1 2 A: SCM-Gd-PEG* 0. 26 88. 38 - 2 D: SCM-Gd (NO PEG) 0. 16 92. 72 - * Used in ‘Seek & Destroy’ Control Release Therapy Abbreviation Key 1. SCM: TAASI Propriety Information 2. A: Autoclaved 3. Gd: Gadolinium Oxide 4. PEG: Polyethylene Glycol Page 8

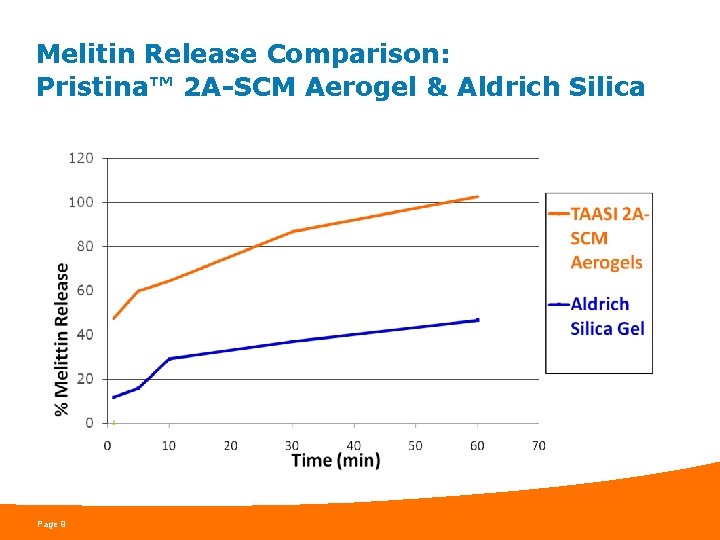
Melitin Release Comparison: Pristina™ 2 A-SCM Aerogel & Aldrich Silica Page 9

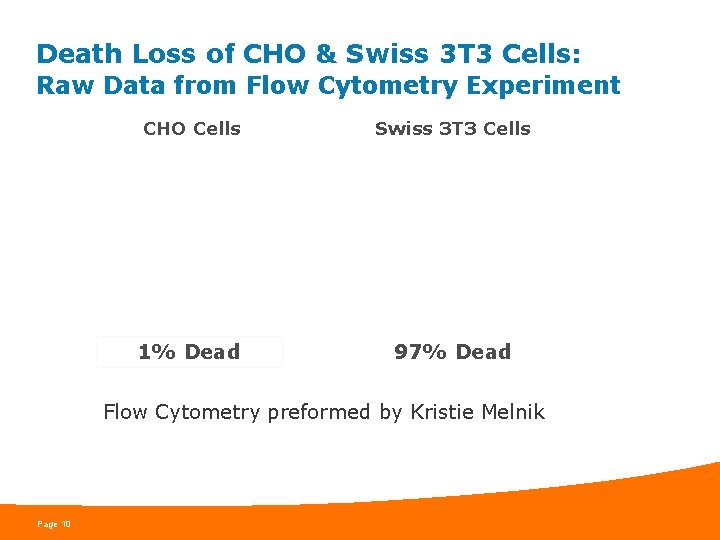
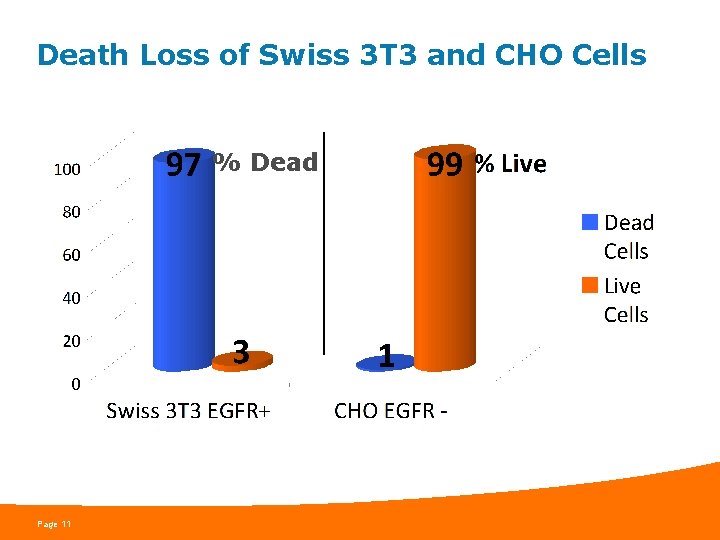
Death Loss of CHO & Swiss 3 T 3 Cells: Raw Data from Flow Cytometry Experiment CHO Cells Swiss 3 T 3 Cells 1% Dead 97% Dead Flow Cytometry preformed by Kristie Melnik Page 10

Death Loss of Swiss 3 T 3 and CHO Cells % Dead Page 11
 Pristina teliska
Pristina teliska Seagel aerogel
Seagel aerogel Targeted youth support islington
Targeted youth support islington Targeted universalism
Targeted universalism Tst joint commission
Tst joint commission Target local hire
Target local hire Targeted local hiring program
Targeted local hiring program Targeted disabilities
Targeted disabilities Targeted local hiring program
Targeted local hiring program Mshda dpa
Mshda dpa Targeted early numeracy intervention program
Targeted early numeracy intervention program Marketing involve engaging directly with carefully targeted
Marketing involve engaging directly with carefully targeted