Development of organisms Biology 122 Genes and Development

Development of organisms Biology 122 Genes and Development

Fig. 19. 1 Cleavage in a frog embryo
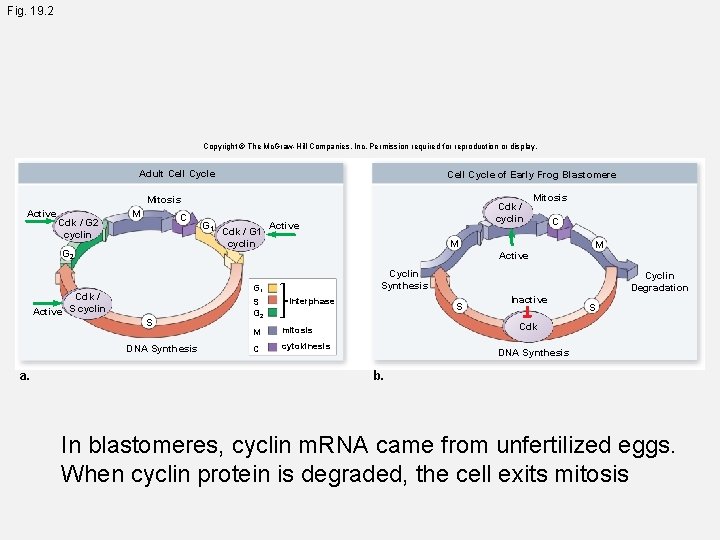
Fig. 19. 2 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Adult Cell Cycle of Early Frog Blastomere Mitosis Active Cdk / G 2 cyclin M C G 2 Cdk / Active S cyclin Cdk / G 1 cyclin Active Mitosis C M Active S S G 2 M Cyclin Synthesis G 1 DNA Synthesis a. G 1 Cdk / cyclin interphase M mitosis C cytokinesis Cyclin Degradation S Inactive S Cdk DNA Synthesis b. In blastomeres, cyclin m. RNA came from unfertilized eggs. When cyclin protein is degraded, the cell exits mitosis
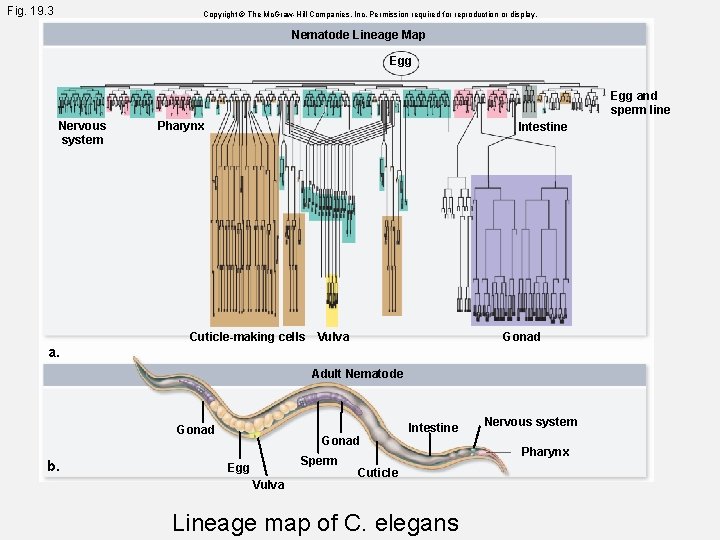
Fig. 19. 3 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Nematode Lineage Map Egg and sperm line Nervous system a. Pharynx Intestine Cuticle-making cells Vulva Gonad Adult Nematode Gonad b. Gonad Sperm Egg Vulva Intestine Cuticle Lineage map of C. elegans Nervous system Pharynx
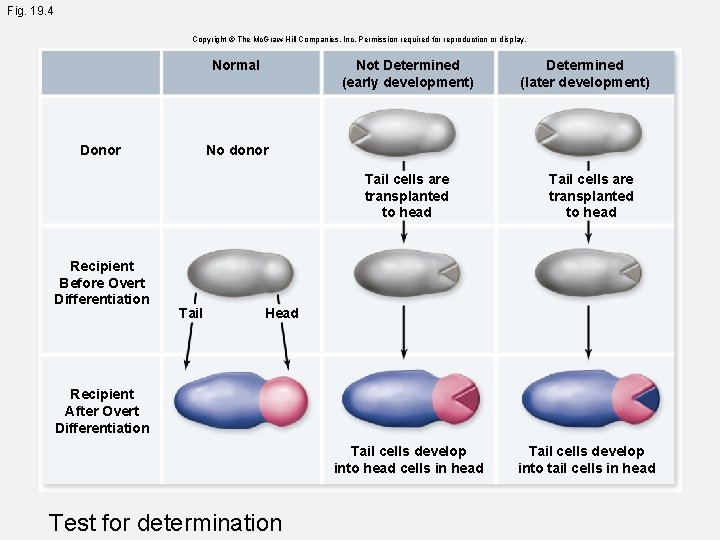
Fig. 19. 4 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Normal Not Determined (early development) No donor Donor Tail cells are transplanted to head Recipient Before Overt Differentiation Determined (later development) Tail cells are transplanted to head Head Recipient After Overt Differentiation Tail cells develop into head cells in head Test for determination Tail cells develop into tail cells in head
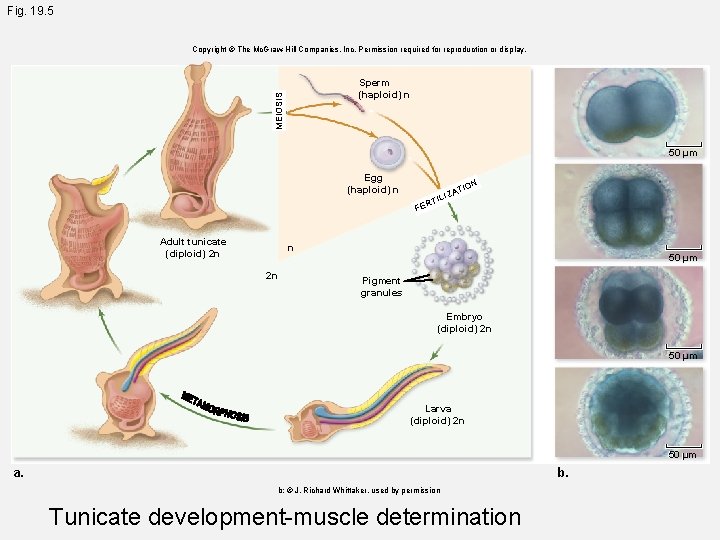
Fig. 19. 5 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. MEIOSIS Sperm (haploid) n 50 µm Egg (haploid) n A ILIZ N TIO RT FE Adult tunicate (diploid) 2 n n 2 n 50 µm Pigment granules Embryo (diploid) 2 n 50 µm Larva (diploid) 2 n 50 µm a. b. b: © J. Richard Whittaker, used by permission Tunicate development-muscle determination
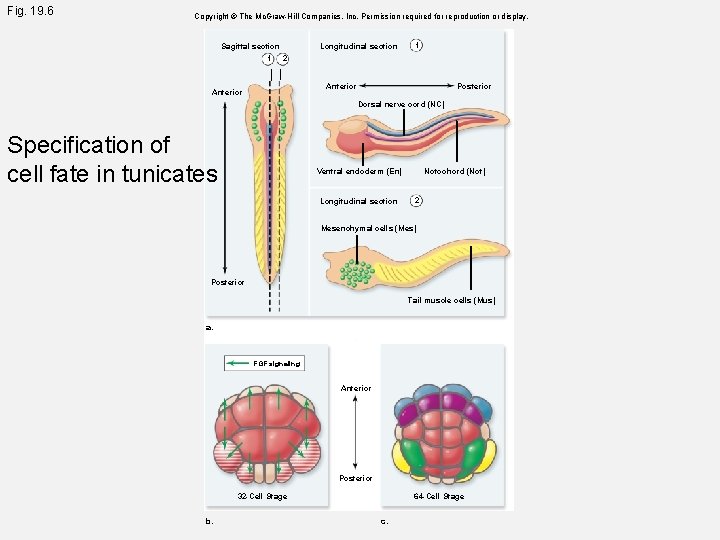
Fig. 19. 6 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Sagittal section 1 Longitudinal section 1 2 Anterior Posterior Dorsal nerve cord (NC) Specification of cell fate in tunicates Notochord (Not) Ventral endoderm (En) Longitudinal section 2 Mesenchymal cells (Mes) Posterior Tail muscle cells (Mus) a. FGF signaling Anterior Posterior 32 -Cell Stage b. 64 -Cell Stage c.

Fig. 19. 7 -1 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. First Step Second Step Cell Types Yes FGF Signal received Yes No Mesenchyme No FGF Signal received Yes No Notochord Macho-1 inherited? a. Cell fate specification in tunicates Muscle Nerve cord
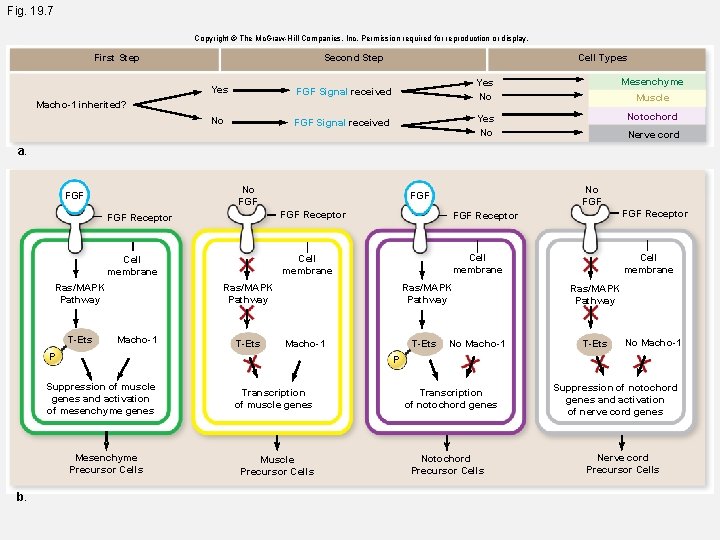
Fig. 19. 7 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. First Step Second Step Cell Types Yes FGF Signal received Yes No Mesenchyme No FGF Signal received Yes No Notochord Macho-1 inherited? Muscle Nerve cord a. No FGF FGF Receptor Cell membrane Ras/MAPK Pathway T-Ets Macho-1 T-Ets Ras/MAPK Pathway T-Ets Macho-1 P No Macho-1 Ras/MAPK Pathway T-Ets No Macho-1 P Suppression of muscle genes and activation of mesenchyme genes Mesenchyme Precursor Cells b. No FGF Transcription of muscle genes Muscle Precursor Cells Transcription of notochord genes Notochord Precursor Cells Suppression of notochord genes and activation of nerve cord genes Nerve cord Precursor Cells
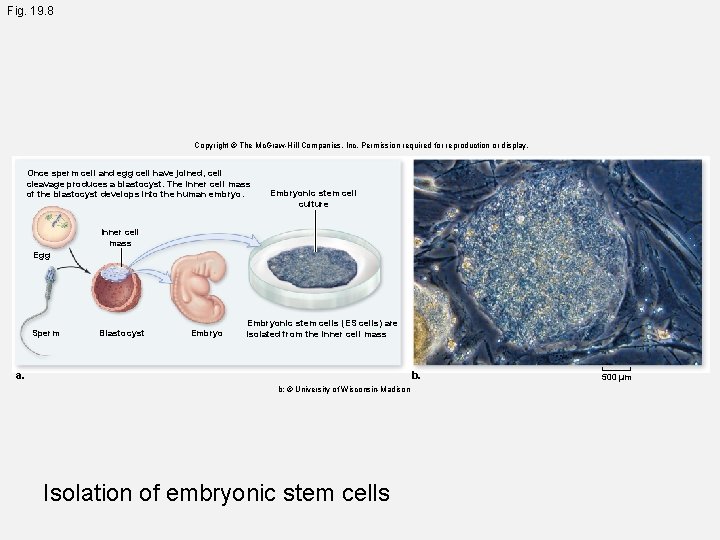
Fig. 19. 8 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Once sperm cell and egg cell have joined, cell cleavage produces a blastocyst. The inner cell mass of the blastocyst develops into the human embryo. Embryonic stem cell culture Inner cell mass Egg Sperm Blastocyst Embryonic stem cells (ES cells) are isolated from the inner cell mass a. b. b: © University of Wisconsin-Madison Isolation of embryonic stem cells 500 µm
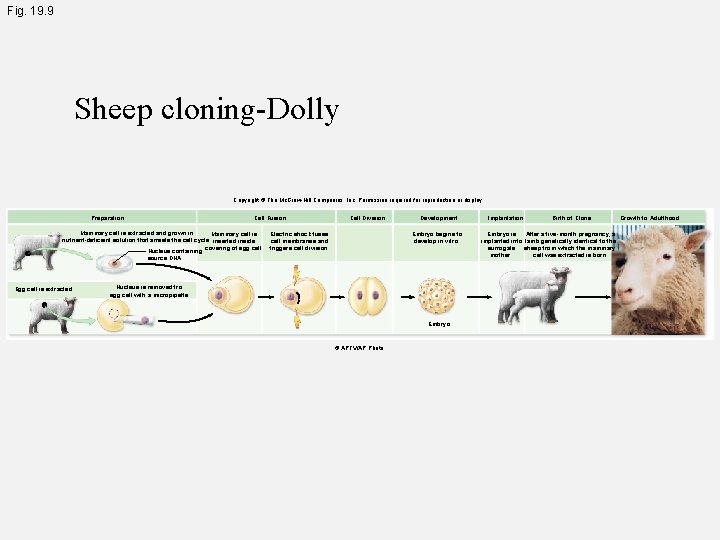
Fig. 19. 9 Sheep cloning-Dolly Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Preparation Cell Fusion Mammary cell is extracted and grown in Mammary cell is nutrient-deficient solution that arrests the cell cycle. inserted inside Nucleus containing covering of egg cell. Cell Division Electric shock fuses cell membranes and triggers cell division. Development Embryo begins to develop in vitro. source DNA Egg cell is extracted. Nucleus is removed fro egg cell with a micropipette. Embryo © APTV/AP Photo Implantation Birth of Clone Embryo is After a five-month pregnancy, a implanted into lamb genetically identical to the surrogate sheep from which the mammary mother. cell was extracted is born. Growth to Adulthood
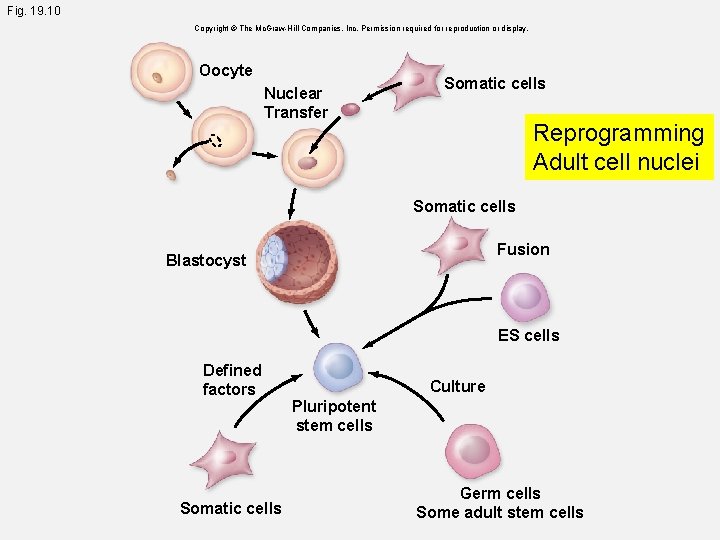
Fig. 19. 10 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Oocyte Nuclear Transfer Somatic cells Reprogramming Adult cell nuclei Somatic cells Fusion Blastocyst ES cells Defined factors Somatic cells Culture Pluripotent stem cells Germ cells Some adult stem cells
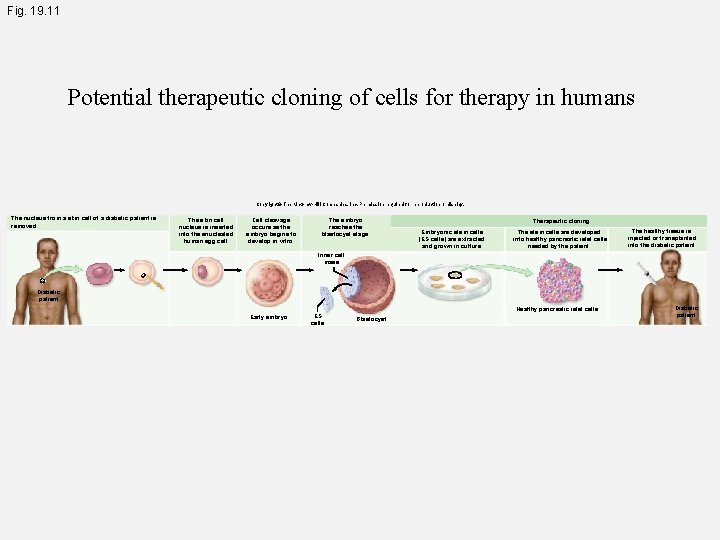
Fig. 19. 11 Potential therapeutic cloning of cells for therapy in humans Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. The nucleus from a skin cell of a diabetic patient is removed. The skin cell nucleus is inserted into the enucleated human egg cell. Cell cleavage occurs as the embryo begins to develop in vitro. The embryo reaches the blastocyst stage. Therapeutic cloning Embryonic stem cells (ES cells) are extracted and grown in culture. The stem cells are developed into healthy pancreatic islet cells needed by the patient. The healthy tissue is injected or transplanted into the diabetic patient. Inner cell mass Diabetic patient Healthy pancreatic islet cells Early embryo ES cells Blastocyst Diabetic patient
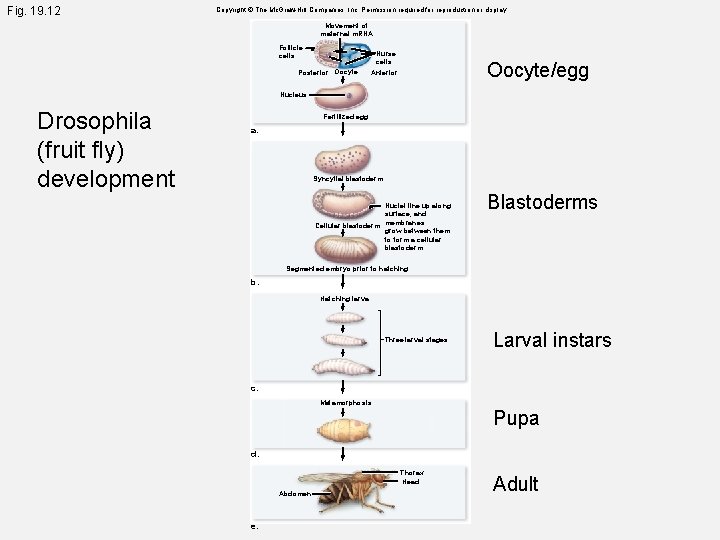
Fig. 19. 12 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Movement of maternal m. RNA Follicle cells Nurse cells Posterior Oocyte/egg Anterior Nucleus Drosophila (fruit fly) development Fertilized egg a. Syncytial blastoderm Nuclei line up along surface, and Cellular blastoderm membranes grow between them to form a cellular blastoderm. Blastoderms Segmented embryo prior to hatching b. Hatching larva Three larval stages Larval instars c. Metamorphosis Pupa d. Thorax Head Abdomen e. Adult
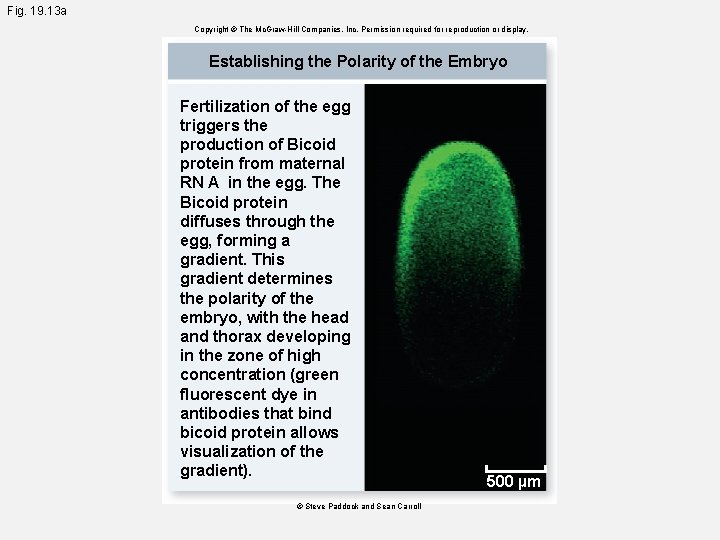
Fig. 19. 13 a Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Establishing the Polarity of the Embryo Fertilization of the egg triggers the production of Bicoid protein from maternal RN A in the egg. The Bicoid protein diffuses through the egg, forming a gradient. This gradient determines the polarity of the embryo, with the head and thorax developing in the zone of high concentration (green fluorescent dye in antibodies that bind bicoid protein allows visualization of the gradient). © Steve Paddock and Sean Carroll 500 µm

Fig. 19. 13 b Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Setting the Stage for Segmentation About 21/2 hours after fertilization, Bicoid protein turns on a series of brief signals from so-called gap genes. The gap proteins act to divide the embryo into large blocks. In this photo, fluorescent dyes in antibodies that bind to the gap proteins Krüppel (orange) and Hunchback (green) make the blocks visible; the region of overlap is yellow. 500 µm © Jim Langeland, Steve Paddock and Sean Carroll

Fig. 19. 13 c Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Laying Down the Fundamental Regions About 0. 5 hr later , the gap genes switch on the “pair-rule” genes, which are each expressed in seven stripes. This is shown for the pair-rule gene hairy. Some pair-rule genes are only required for even-numbered segments while others are only required for odd numbered segments. 500 µm © Jim Langeland, Steve Paddock and Sean Carroll
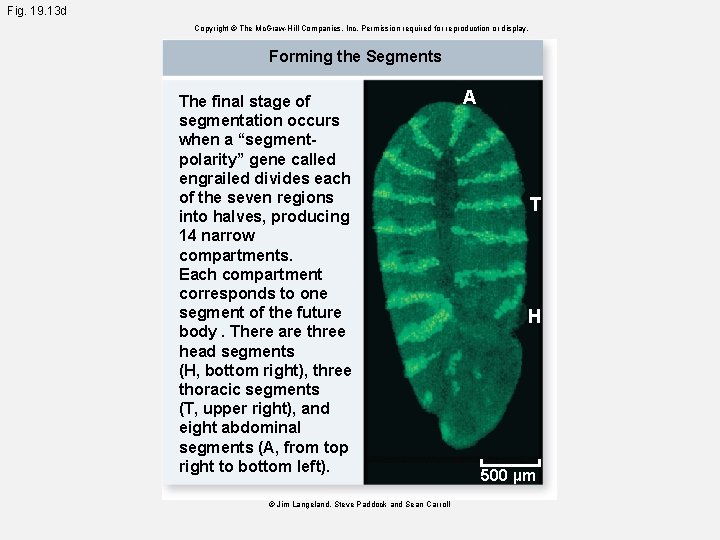
Fig. 19. 13 d Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Forming the Segments The final stage of segmentation occurs when a “segmentpolarity” gene called engrailed divides each of the seven regions into halves, producing 14 narrow compartments. Each compartment corresponds to one segment of the future body. There are three head segments (H, bottom right), three thoracic segments (T, upper right), and eight abdominal segments (A, from top right to bottom left). © Jim Langeland, Steve Paddock and Sean Carroll A T H 500 µm
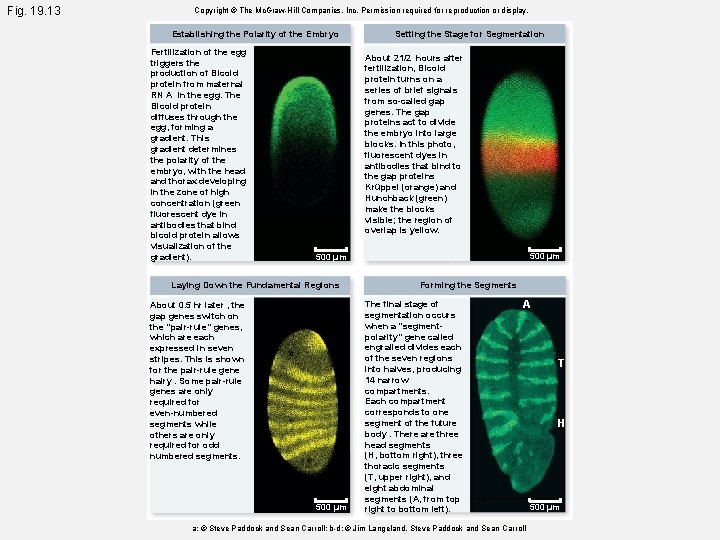
Fig. 19. 13 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Establishing the Polarity of the Embryo Fertilization of the egg triggers the production of Bicoid protein from maternal RN A in the egg. The Bicoid protein diffuses through the egg, forming a gradient. This gradient determines the polarity of the embryo, with the head and thorax developing in the zone of high concentration (green fluorescent dye in antibodies that bind bicoid protein allows visualization of the gradient). Setting the Stage for Segmentation About 21/2 hours after fertilization, Bicoid protein turns on a series of brief signals from so-called gap genes. The gap proteins act to divide the embryo into large blocks. In this photo, fluorescent dyes in antibodies that bind to the gap proteins Krüppel (orange) and Hunchback (green) make the blocks visible; the region of overlap is yellow. 500 µm Laying Down the Fundamental Regions About 0. 5 hr later , the gap genes switch on the “pair-rule” genes, which are each expressed in seven stripes. This is shown for the pair-rule gene hairy. Some pair-rule genes are only required for even-numbered segments while others are only required for odd numbered segments. 500 µm Forming the Segments The final stage of segmentation occurs when a “segmentpolarity” gene called engrailed divides each of the seven regions into halves, producing 14 narrow compartments. Each compartment corresponds to one segment of the future body. There are three head segments (H, bottom right), three thoracic segments (T, upper right), and eight abdominal segments (A, from top right to bottom left). A a: © Steve Paddock and Sean Carroll; b-d: © Jim Langeland, Steve Paddock and Sean Carroll T H 500 µm
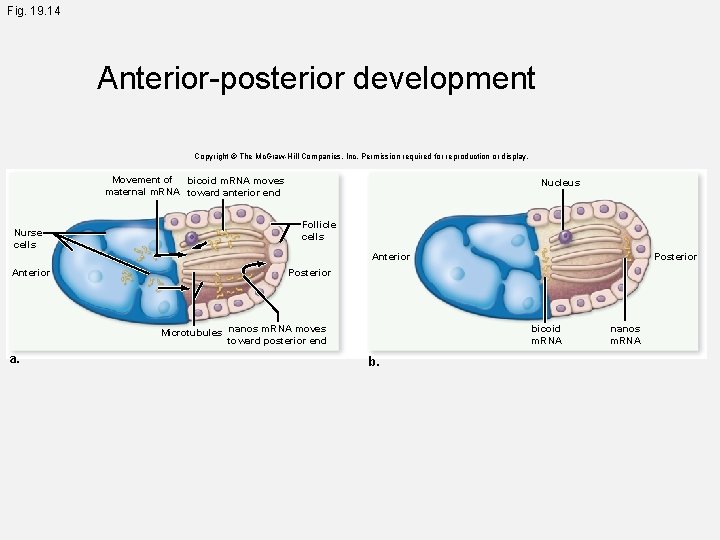
Fig. 19. 14 Anterior-posterior development Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Movement of bicoid m. RNA moves maternal m. RNA toward anterior end Nurse cells Nucleus Follicle cells Anterior Posterior Microtubules nanos m. RNA moves toward posterior end a. Posterior bicoid m. RNA b. nanos m. RNA
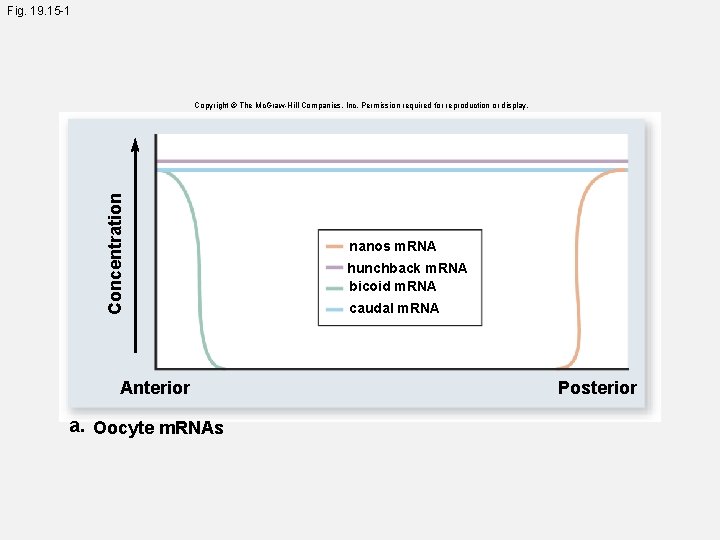
Fig. 19. 15 -1 Concentration Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Anterior a. Oocyte m. RNAs nanos m. RNA hunchback m. RNA bicoid m. RNA caudal m. RNA Posterior
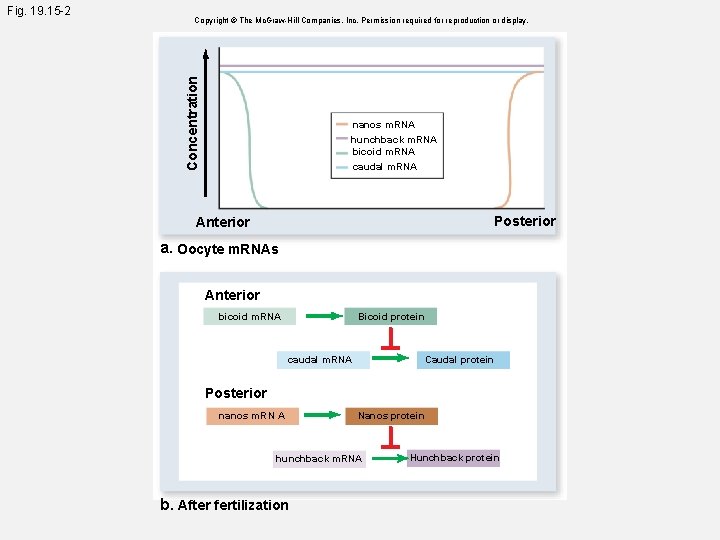
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Concentration Fig. 19. 15 -2 nanos m. RNA hunchback m. RNA bicoid m. RNA caudal m. RNA Posterior Anterior a. Oocyte m. RNAs Anterior bicoid m. RNA Bicoid protein caudal m. RNA Caudal protein Posterior nanos m. RN A Nanos protein hunchback m. RNA b. After fertilization Hunchback protein
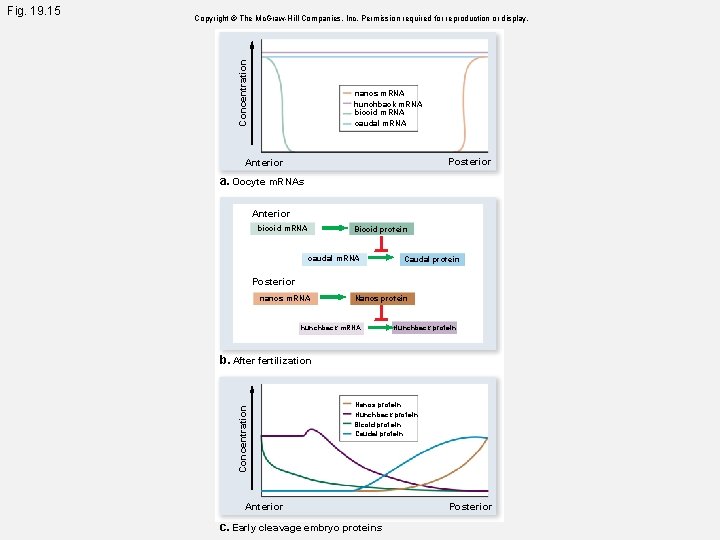
Concentration Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. nanos m. RNA hunchback m. RNA bicoid m. RNA caudal m. RNA Posterior Anterior a. Oocyte m. RNAs Anterior bicoid m. RNA Bicoid protein caudal m. RNA Caudal protein Posterior nanos m. RNA Nanos protein hunchback m. RNA Hunchback protein b. After fertilization Concentration Fig. 19. 15 Nanos protein Hunchback protein Bicoid protein Caudal protein Anterior c. Early cleavage embryo proteins Posterior
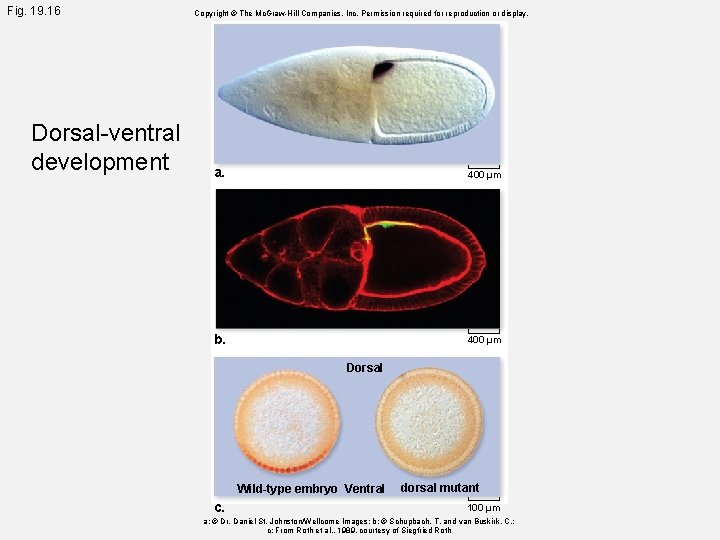
Fig. 19. 16 Dorsal-ventral development Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. a. 400 µm b. 400 µm Dorsal Wild-type embryo Ventral c. dorsal mutant 100 µm a: © Dr. Daniel St. Johnston/Wellcome Images; b: © Schupbach, T. and van Buskirk, C. ; c: From Roth et al. , 1989, courtesy of Siegfried Roth

Fig. 19. 17

Fig. 19. 18 a Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Drosophila HOM Chromosomes Drosophila HOM genes Antennapedia complex Bithorax complex lab pb Dfd Scr Antp Ubx abd-A abd-B Head Thorax Abdomen Fruit fly embryo Fruit fly a.
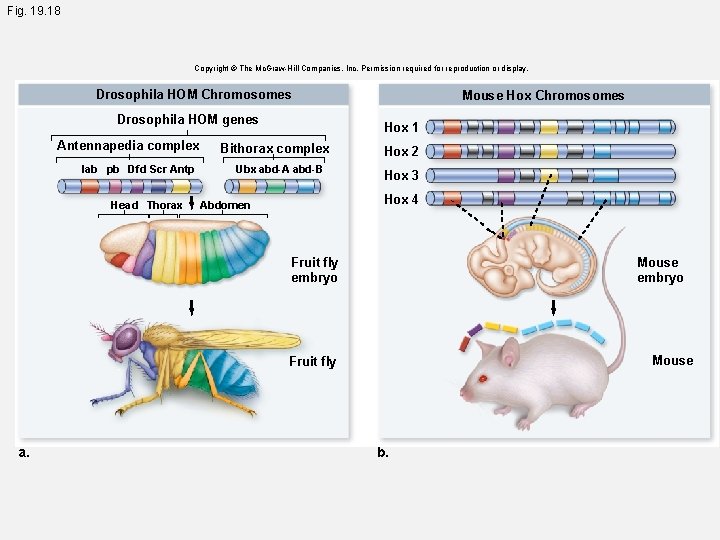
Fig. 19. 18 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Drosophila HOM Chromosomes Drosophila HOM genes Antennapedia complex lab pb Dfd Scr Antp Head Thorax Mouse Hox Chromosomes Hox 1 Bithorax complex Ubx abd-A abd-B Hox 2 Hox 3 Hox 4 Abdomen Fruit fly embryo Mouse Fruit fly a. b.

SEM of He. La cell undergoing apoptosis From ATCC photo contest, 2011
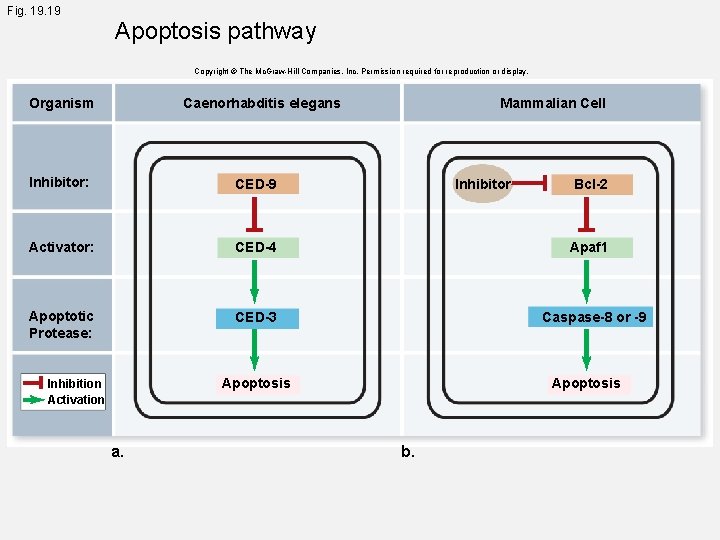
Fig. 19 Apoptosis pathway Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Organism Caenorhabditis elegans Inhibitor: CED-9 Activator: CED-4 Apoptotic Protease: CED-3 Mammalian Cell Inhibitor Apaf 1 Caspase-8 or -9 Apoptosis Inhibition Activation a. Bcl-2 Apoptosis b.
- Slides: 29