Development of Atomic Structure How long have people







- Slides: 15

Development of Atomic Structure How long have people been interested in understanding matter and its structure? A. Thousands of years B. Hundreds of years C. A few years D. Never • Answer: A

Democritus (ca. 450 BC)

Ancient Philosophy • • Who: Aristotle & Democritus When: More than 2000 years ago Where: Greece What: Aristotle believed in 4 elements: Earth, Air, Fire, and Water. Democritus believed that matter was made of small particles he named “atoms”. • Why: Aristotle and Democritus used observation and inference to explain the existence of everything.

Alchemists • • Who: European Scientists When: 800 – 900 years ago Where: Europe What: Their work developed into what is now modern chemistry. • Why: Trying to change ordinary materials into gold.

Alchemic Symbols

John Dalton (1766 -1844) • Based his work on Lavoisier (Law of Convservation of Mass)

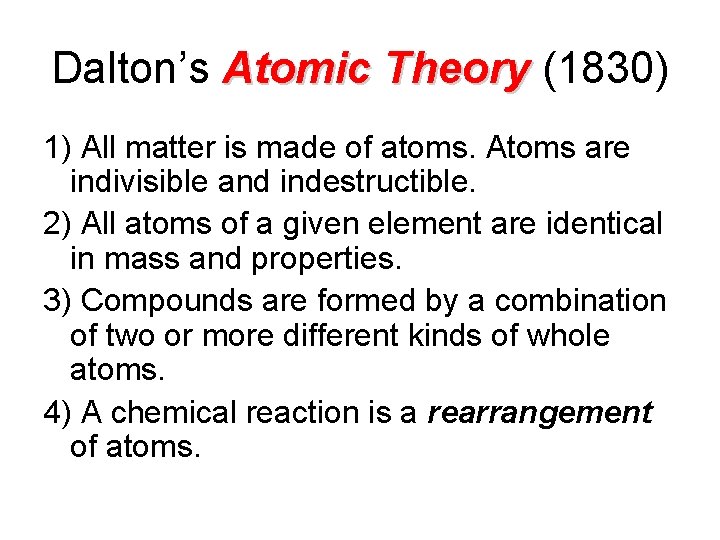
Dalton’s Atomic Theory (1830) 1) All matter is made of atoms. Atoms are indivisible and indestructible. 2) All atoms of a given element are identical in mass and properties. 3) Compounds are formed by a combination of two or more different kinds of whole atoms. 4) A chemical reaction is a rearrangement of atoms.

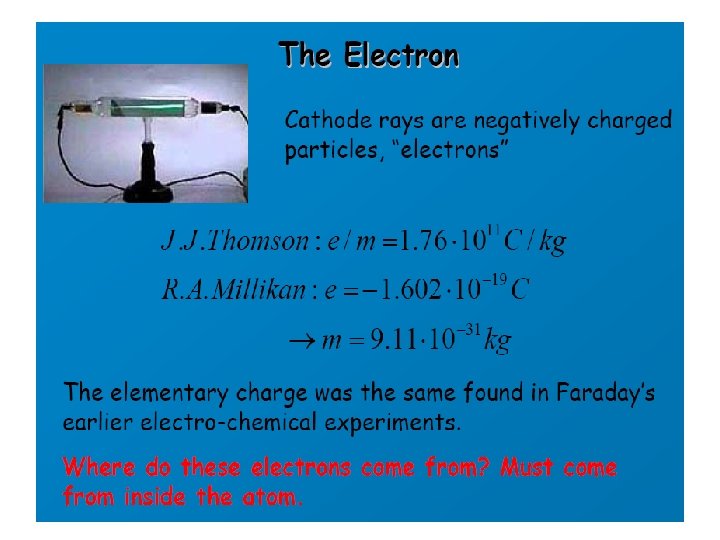
J. J. Thomson (1856 -1940) • Discovery of ELECTRON

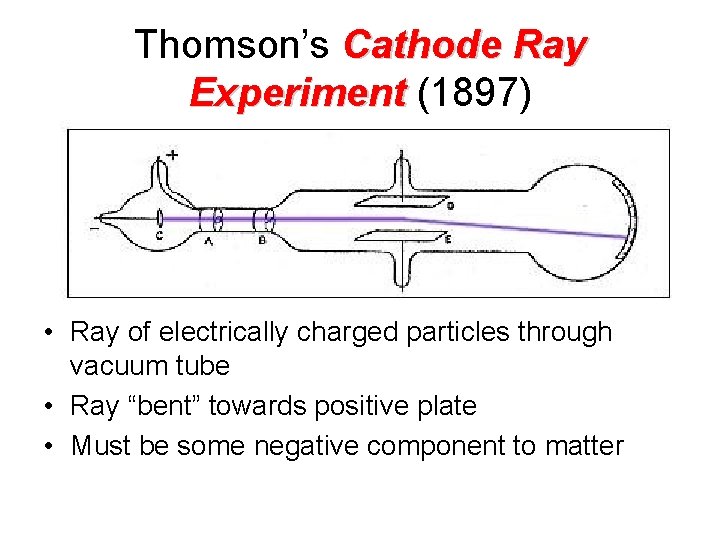
Thomson’s Cathode Ray Experiment (1897) • Ray of electrically charged particles through vacuum tube • Ray “bent” towards positive plate • Must be some negative component to matter



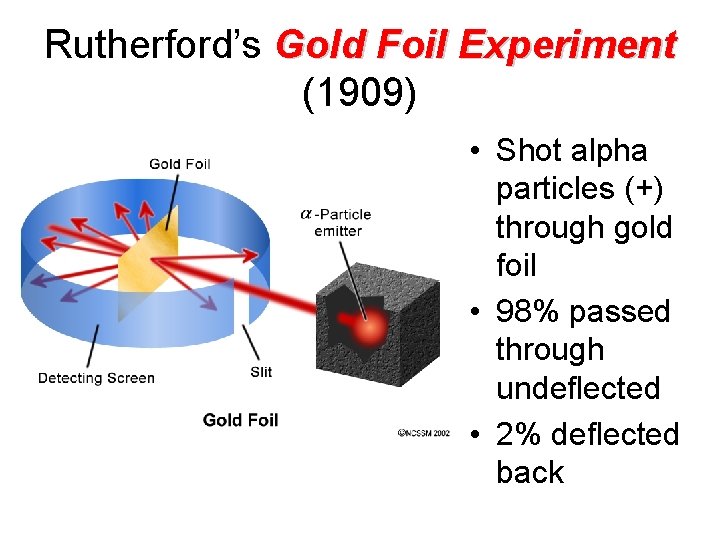
Ernest Rutherford (1871 -1937) • Discovery of NUCLEUS

Rutherford’s Gold Foil Experiment (1909) • Shot alpha particles (+) through gold foil • 98% passed through undeflected • 2% deflected back

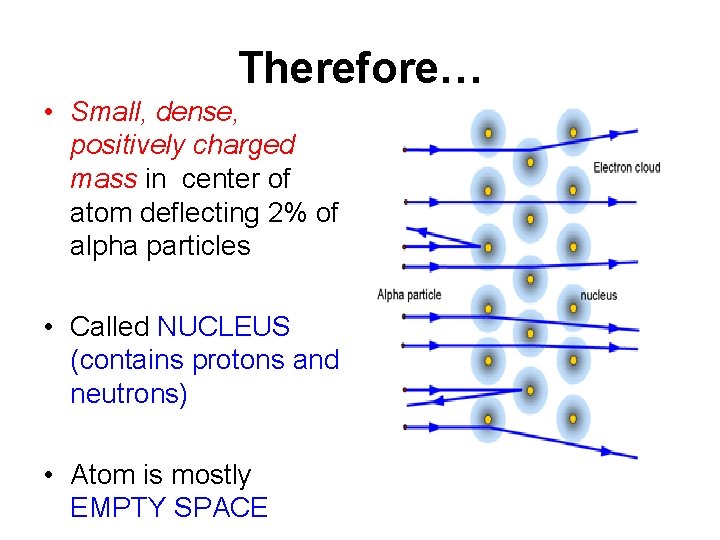
Therefore… • Small, dense, positively charged mass in center of atom deflecting 2% of alpha particles • Called NUCLEUS (contains protons and neutrons) • Atom is mostly EMPTY SPACE

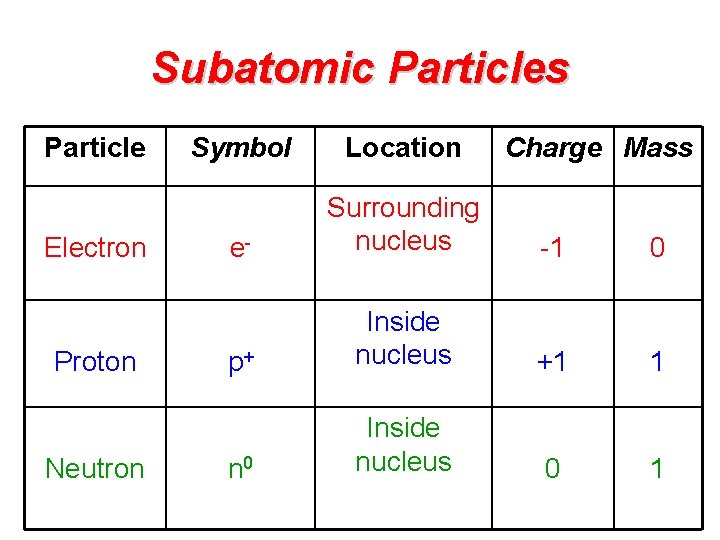
Subatomic Particles Particle Electron Proton Neutron Symbol Location Charge Mass e- Surrounding nucleus -1 0 p+ Inside nucleus +1 1 n 0 Inside nucleus 0 1