Development of Atomic Models Where are the electrons



- Slides: 12

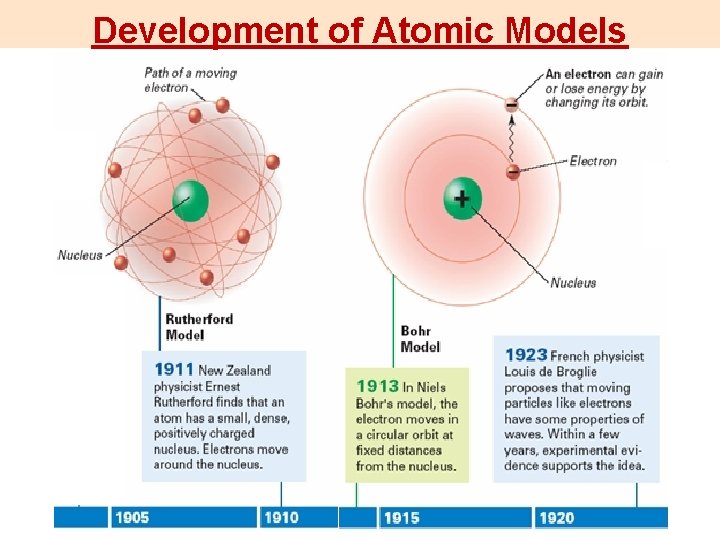
Development of Atomic Models

Where are the electrons exactly? propeller has equal probability of being anywhere in the blurry region, but… …you cannot tell its exact location at any instant.

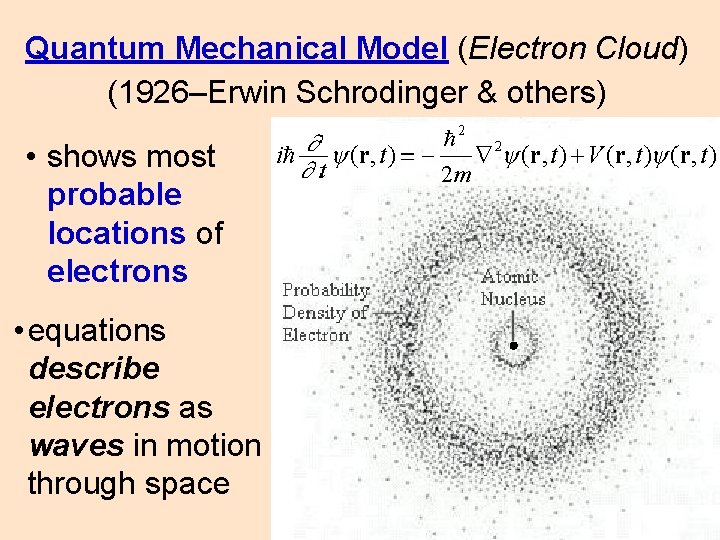
Quantum Mechanical Model (Electron Cloud) (1926–Erwin Schrodinger & others) • shows most probable locations of electrons • equations describe electrons as waves in motion through space

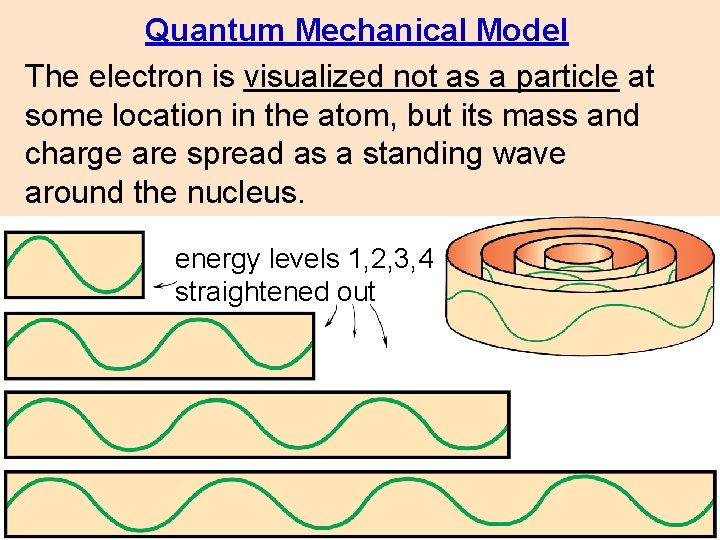
Quantum Mechanical Model The electron is visualized not as a particle at some location in the atom, but its mass and charge are spread as a standing wave around the nucleus. energy levels 1, 2, 3, 4 straightened out

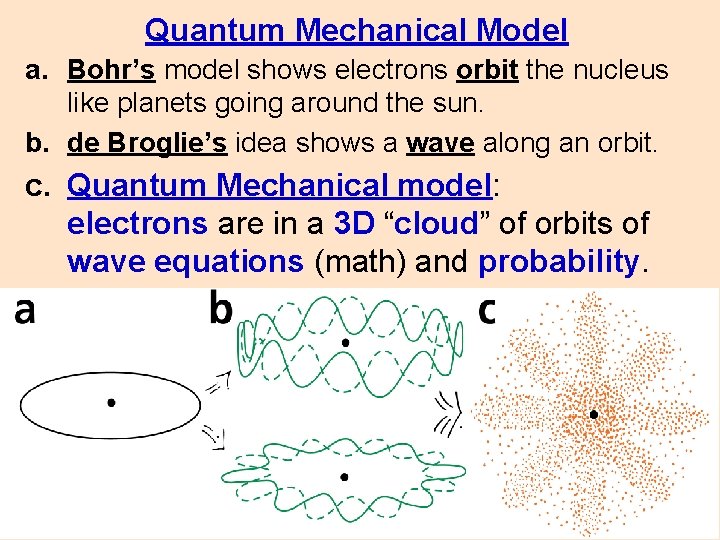
Quantum Mechanical Model a. Bohr’s model shows electrons orbit the nucleus like planets going around the sun. b. de Broglie’s idea shows a wave along an orbit. c. Quantum Mechanical model: electrons are in a 3 D “cloud” of orbits of wave equations (math) and probability.

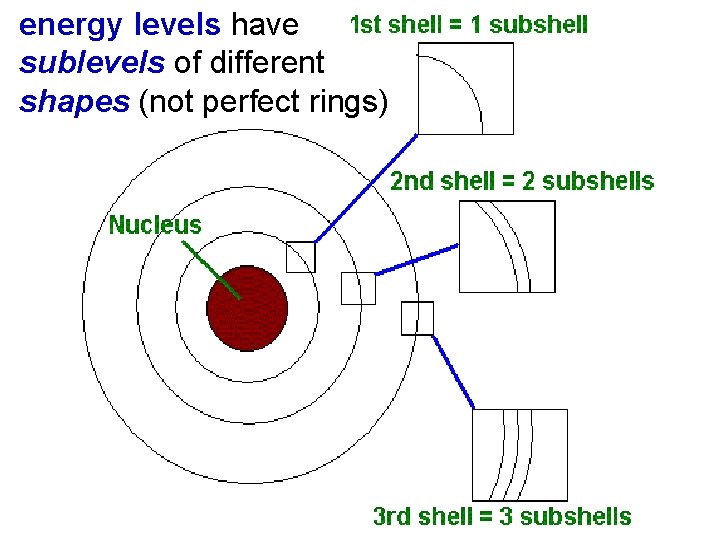
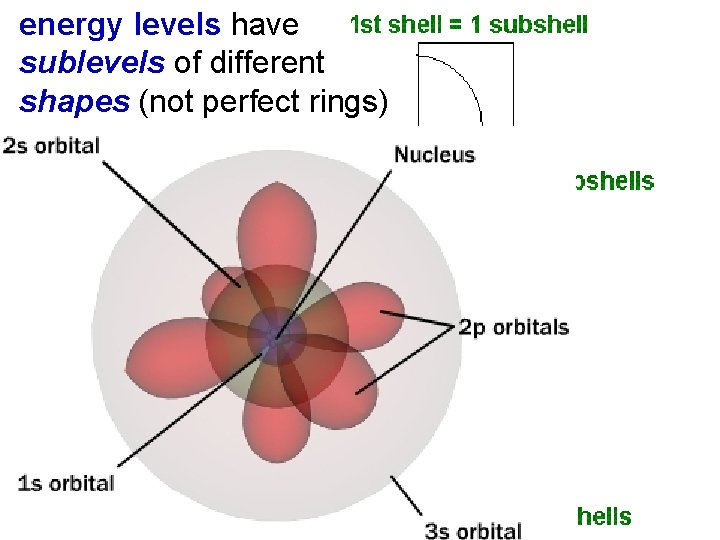
5. 2 levels have Atomic Orbitals energy sublevels of different shapes (not perfect rings) Atomic Orbitals energy levels have sublevels of different shapes (not perfect rings like Bohr proposed)

5. 2 levels have Atomic Orbitals energy sublevels of different shapes (not perfect rings) Atomic Orbitals energy levels have sublevels of different shapes (not perfect rings like Bohr proposed)

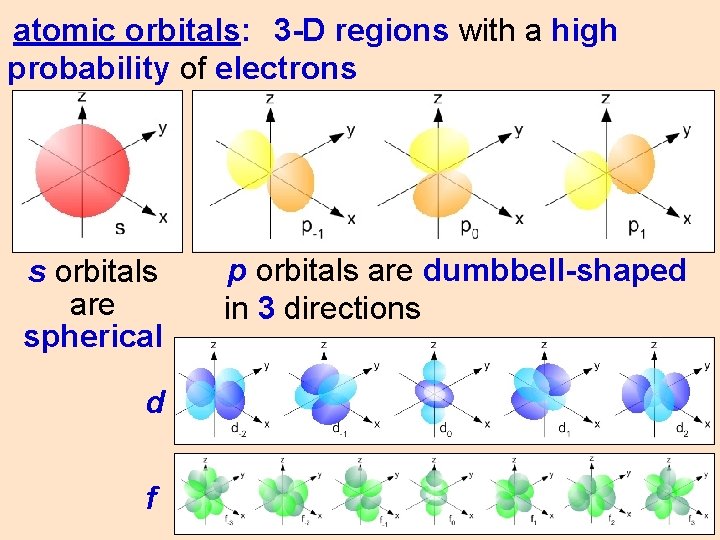
atomic orbitals: 3 -D regions with a high probability of electrons s orbitals are spherical d f p orbitals are dumbbell-shaped in 3 directions

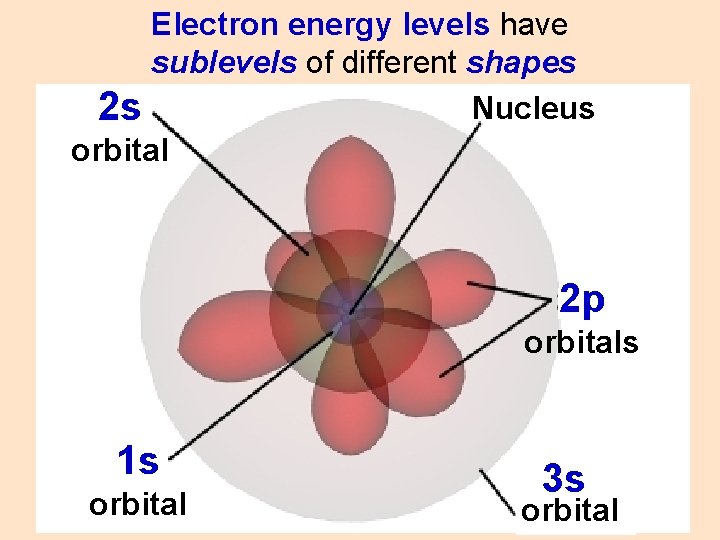
Electron energy levels have sublevels of different shapes Nucleus 2 s orbital 2 p orbitals 1 s orbital 3 s orbital

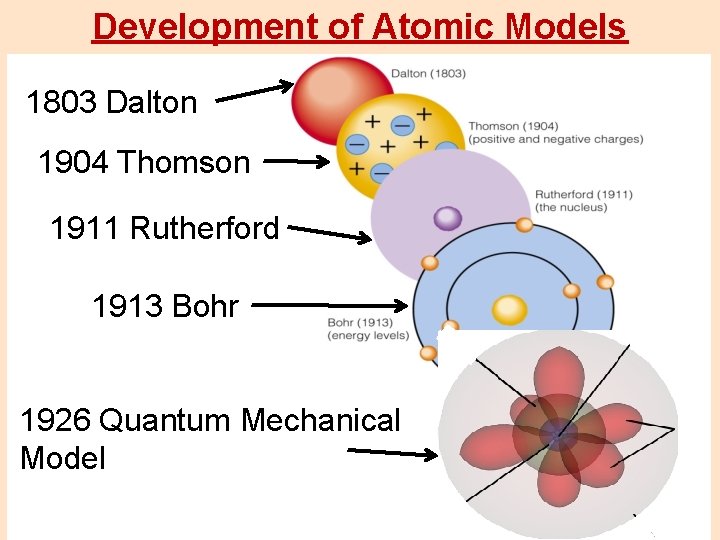
Development of Atomic Models 1803 Dalton 1904 Thomson 1911 Rutherford 1913 Bohr 1926 Quantum Mechanical Model

Quick Quiz! 1. What does the quantum mechanical model describe about atoms? A. the probable locations of electrons in atoms B. the precise locations of electrons in atoms C. the number of electrons in an atom D. how crazy chemistry is

Quick Quiz. 2. What do orbitals (s, p, d, f) tell us about the sublevel of an electron? A. the amount of energy in each electron B. the number of electrons in each sublevel C. the shape of the region they occupy D. that my brain feels mushy now