Development of Atomic Models Indivisible Identical React in
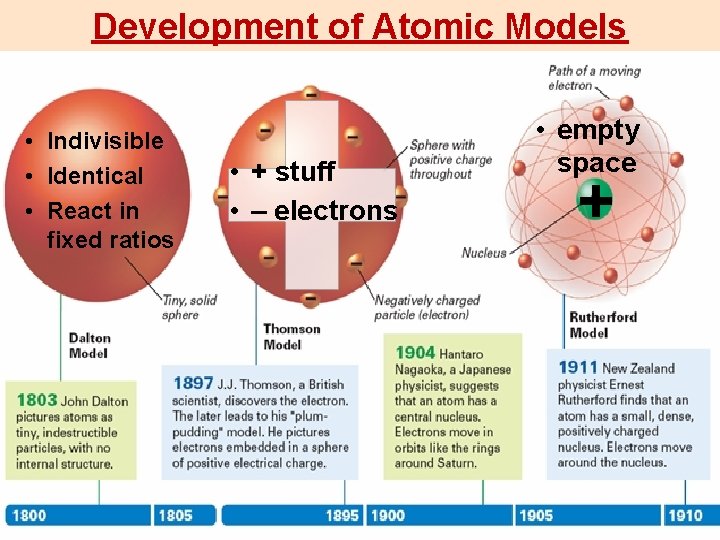
Development of Atomic Models • Indivisible • Identical • React in fixed ratios • + stuff • – electrons • empty space +
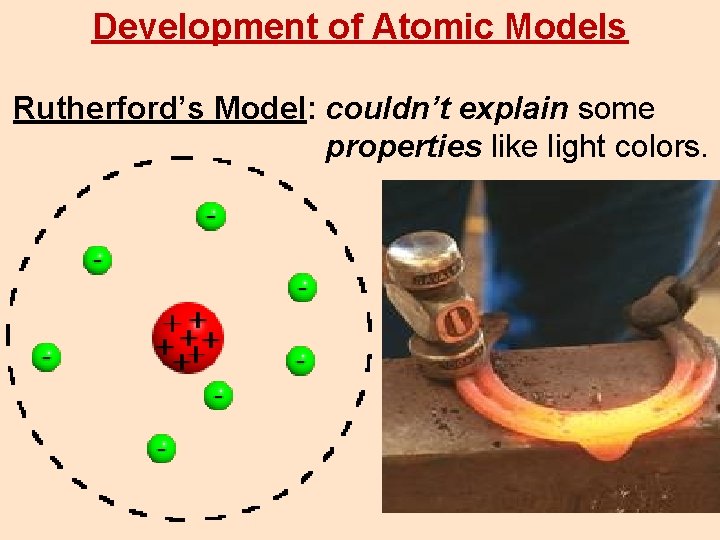
Development of Atomic Models Rutherford’s Model: couldn’t explain some properties like light colors.
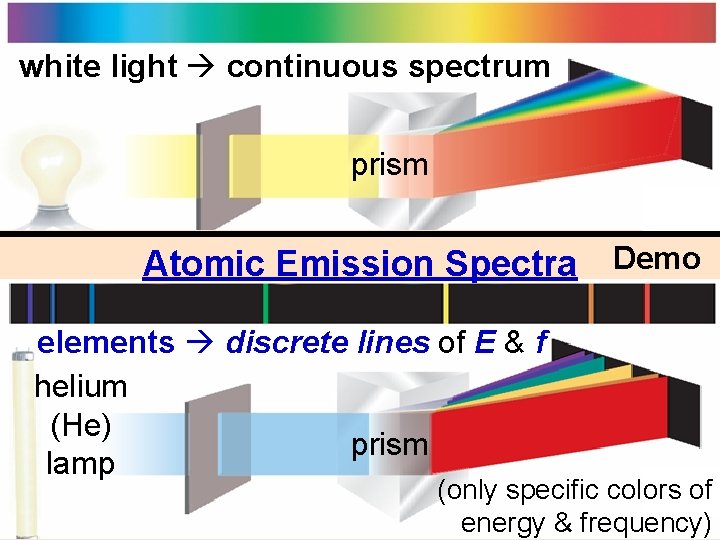
white light continuous spectrum prism Atomic Emission Spectra Demo elements discrete lines of E & f helium (He) prism lamp (only specific colors of energy & frequency)
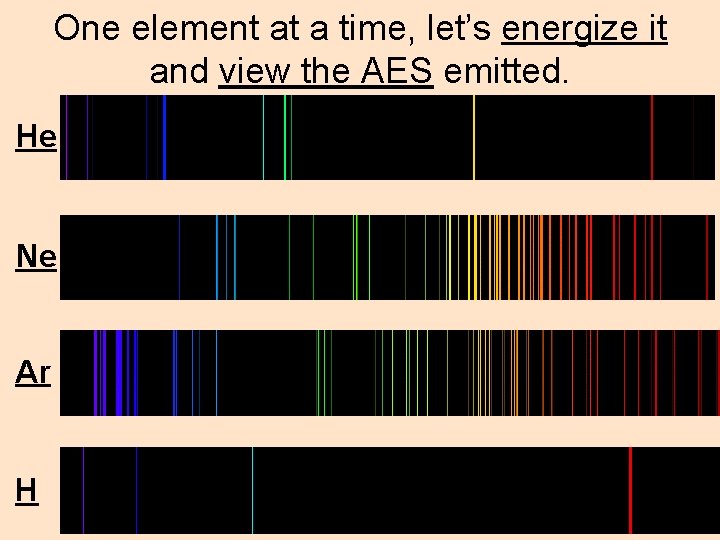
One element at a time, let’s energize it and view the AES emitted. He Ne Ar H
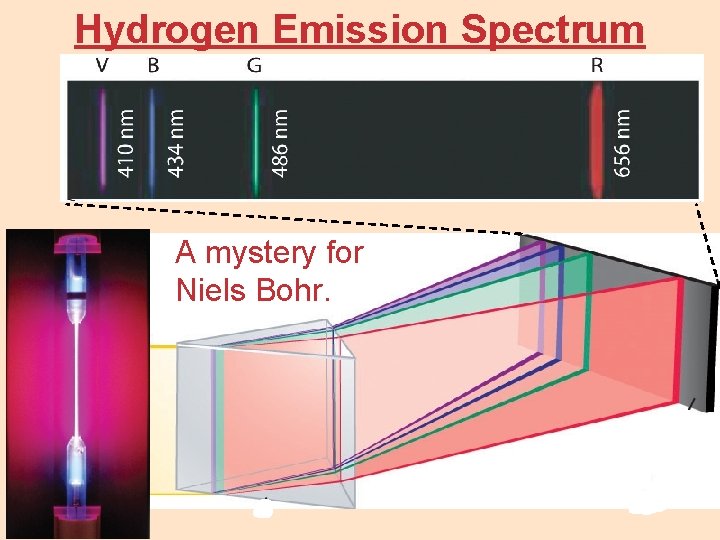
Hydrogen Emission Spectrum A mystery for Niels Bohr.
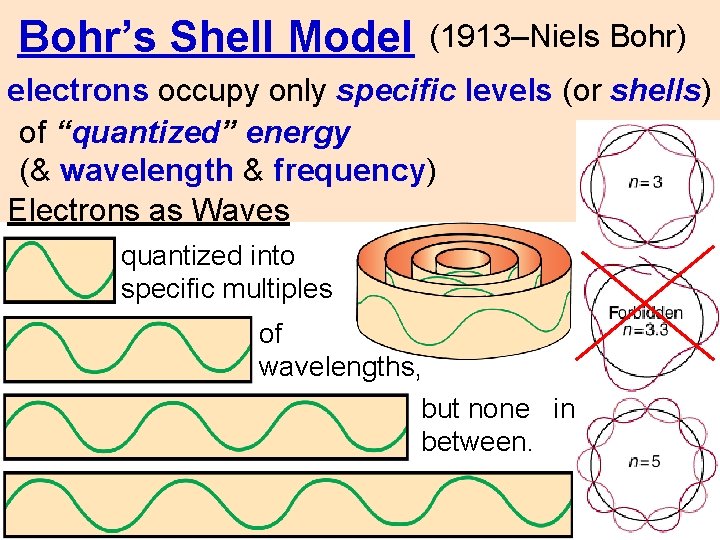
Bohr’s Shell Model (1913–Niels Bohr) electrons occupy only specific levels (or shells) of “quantized” energy (& wavelength & frequency) Electrons as Waves quantized into specific multiples of wavelengths, but none in between.
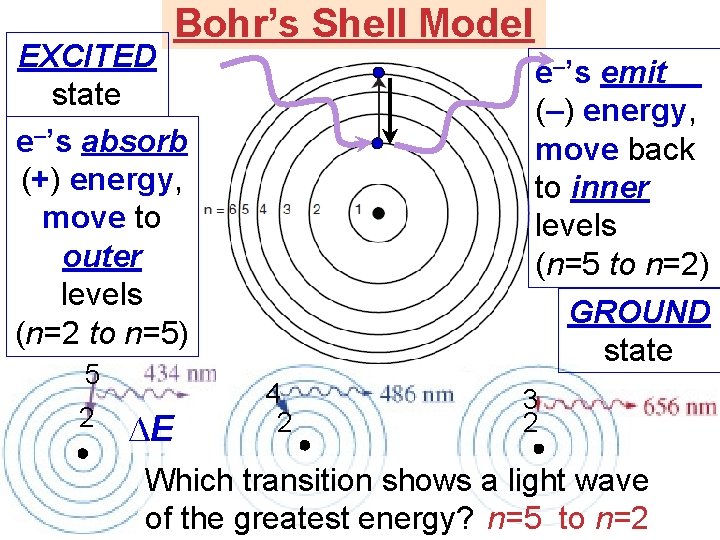
Bohr’s Shell Model EXCITED state e–’s absorb (+) energy, move to outer levels (n=2 to n=5) 5 2 ∆E e–’s emit (–) energy, move back to inner levels (n=5 to n=2) GROUND state 4 2 3 2 Which transition shows a light wave of the greatest energy? n=5 to n=2
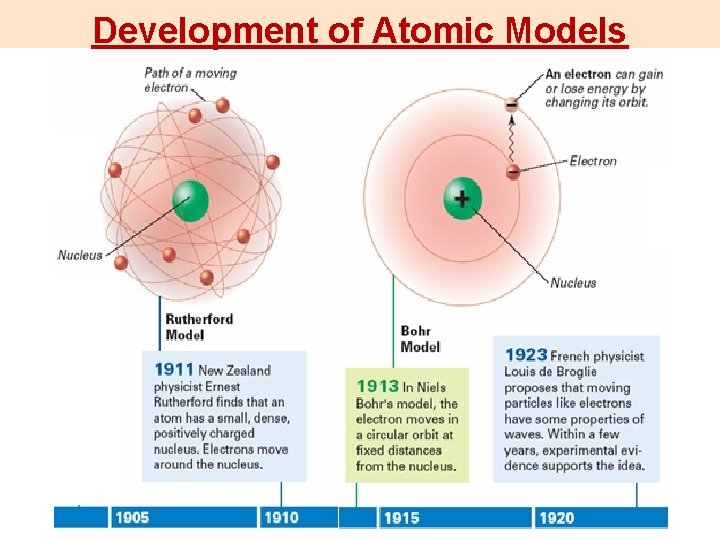
Development of Atomic Models
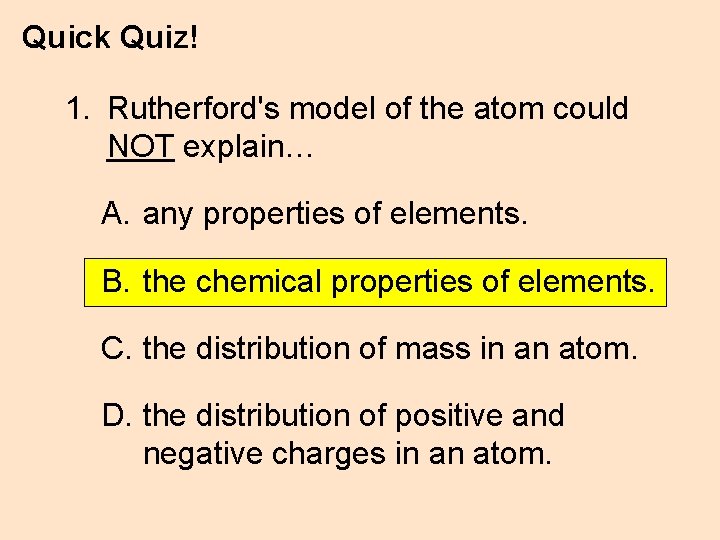
Quick Quiz! 1. Rutherford's model of the atom could NOT explain… A. any properties of elements. B. the chemical properties of elements. C. the distribution of mass in an atom. D. the distribution of positive and negative charges in an atom.

Quick Quiz. 2. Bohr's model of the atom proposed that electrons are found… A. embedded in a sphere of positive charge. B. in fixed positions surrounding the nucleus. C. in fixed orbits of specific energy. D. orbiting the nucleus in a single fixed circular path.

Quick Quiz. 3. The lines in the emission spectrum for an element are caused by… A. the movement of electrons from lower up to higher energy levels. B. the movement of electrons from higher down to lower energy levels. C. the electrons in energy levels in the ground state. D. the electron locations of an atom.
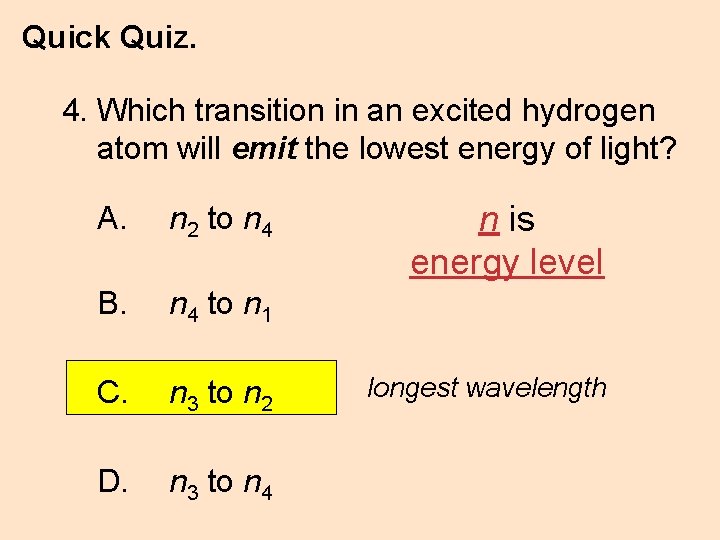
Quick Quiz. 4. Which transition in an excited hydrogen atom will emit the lowest energy of light? A. n 2 to n 4 B. n 4 to n 1 C. n 3 to n 2 D. n 3 to n 4 n is energy level longest wavelength
- Slides: 12