Development of a Quantification Method to Specific AntiNS




- Slides: 15

Development of a Quantification Method to Specific Anti-NS 3 Antibodies against BVDV using a Blocking ELISA Stephan Guillossou 1, 2, Daniel Thomson 1, Cindy Thomson 2 1 Kansas State University, College of Veterinary Medicine, Department of Clinical Science, Manhattan, KS, USA; Corporation, Manhattan, KS, USA; 2 Synbiotics

Introduction BVD Ab Controlling BVDV Identify the best cost effective model European epidemiological models for BVDV control Identify herds harboring PI animals (sentinel animals) Inside these herds, Identify and Remove PI animals Difficulties to adapt in area with use of vaccination needs differentiation between vaccinated induced Abs and virus infection induced Abs (Presence of PI animal)

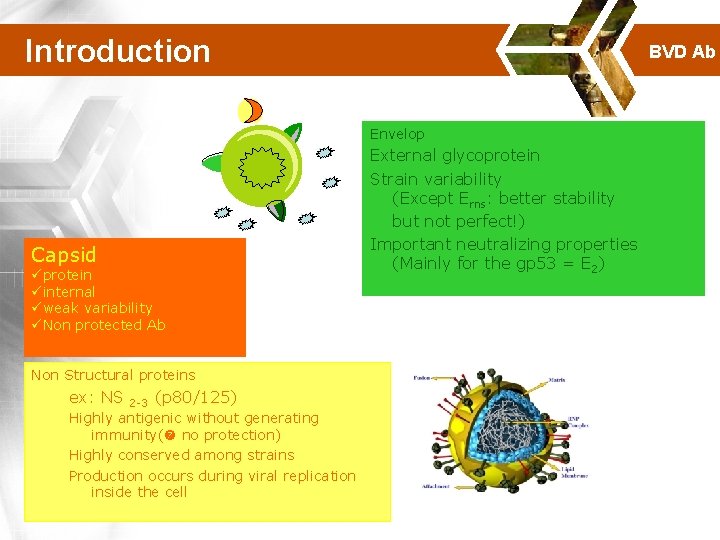
Introduction BVD Ab Envelop Capsid üprotein üinternal üweak variability üNon protected Ab Non Structural proteins ex: NS 2 -3 (p 80/125) Highly antigenic without generating immunity( no protection) Highly conserved among strains Production occurs during viral replication inside the cell External glycoprotein Strain variability (Except Erns: better stability but not perfect!) Important neutralizing properties (Mainly for the gp 53 = E 2)

Objective BVD Ab Sero Neutralizing Test (SNT) measures only antibodies with seroneutralizing properties Western blot are specific to antibody subpopulations Currently, no existing standardized method to quantify subpopulation of antibodies without SN properties Objective of the study: Development of a quantification method to titer anti-NS 3 antibody subpopulation Commercial ELISA SERELISA® BVD p 80 Ab Mono Blocking Synbiotics® Europe, France

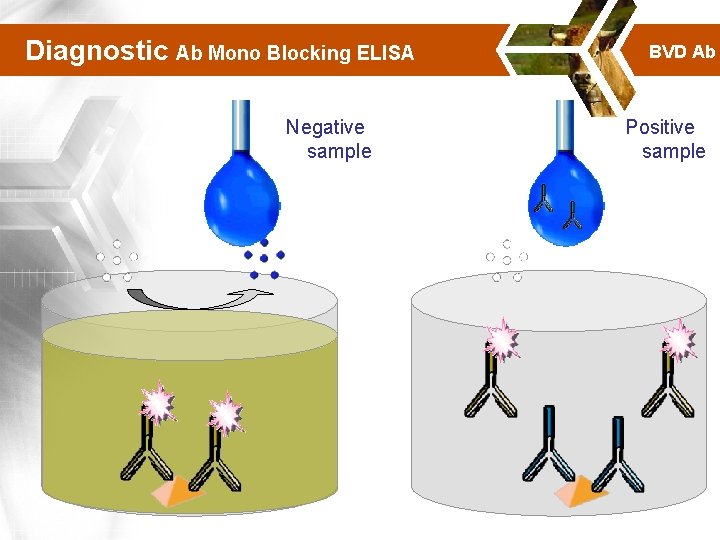
Diagnostic Ab Mono Blocking ELISA Negative sample BVD Ab Positive sample

Objectives & limits/opportunities BVD Ab Objectives: to develop a quantitative serum antibody test for BVDV for a subpopulation of antibodies with a commercially available test Limits/opportunities: Blocking ELISA are specific of only a sub population of antibody targeting a specific epitope of an antigen But blocking ELISA usually have less linearity than indirect ELISA blocking indirect

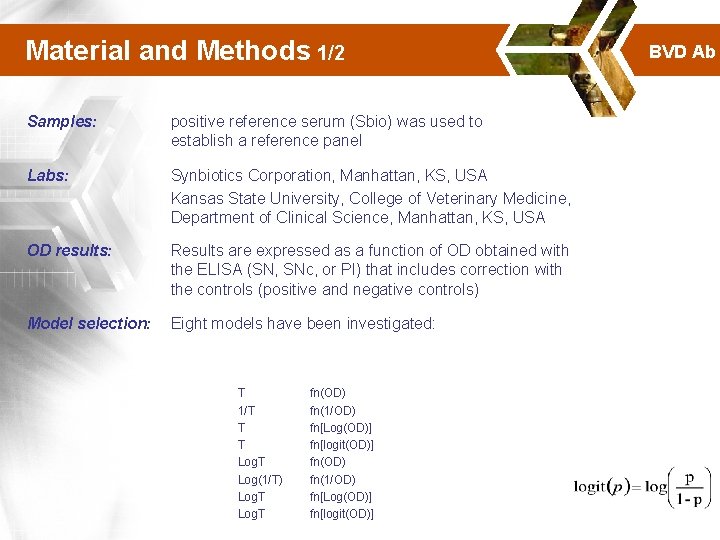
Material and Methods 1/2 Samples: positive reference serum (Sbio) was used to establish a reference panel Labs: Synbiotics Corporation, Manhattan, KS, USA Kansas State University, College of Veterinary Medicine, Department of Clinical Science, Manhattan, KS, USA OD results: Results are expressed as a function of OD obtained with the ELISA (SN, SNc, or PI) that includes correction with the controls (positive and negative controls) Model selection: Eight models have been investigated: T 1/T T T Log(1/T) Log. T fn(OD) fn(1/OD) fn[Log(OD)] fn[logit(OD)] BVD Ab

Material and Methods 2/2 Methods: Model development and selection For each of the previous models, graphical and mathematical pertinence of the models are assessed by interpretation of coefficient of determination R 2 and residual analysis Sample dilution protocol: Reference serum was used at the following dilutions (final dilutions into wells: 1/10, 1/50, 1/100, 1/500, 1/1000, 1/5000, 1/10000 Measures were repeated four times Statistical analysis: R 2. 7. 2 SPSS for Windows ver. 16. 0 Excel ver 2003 BVD Ab

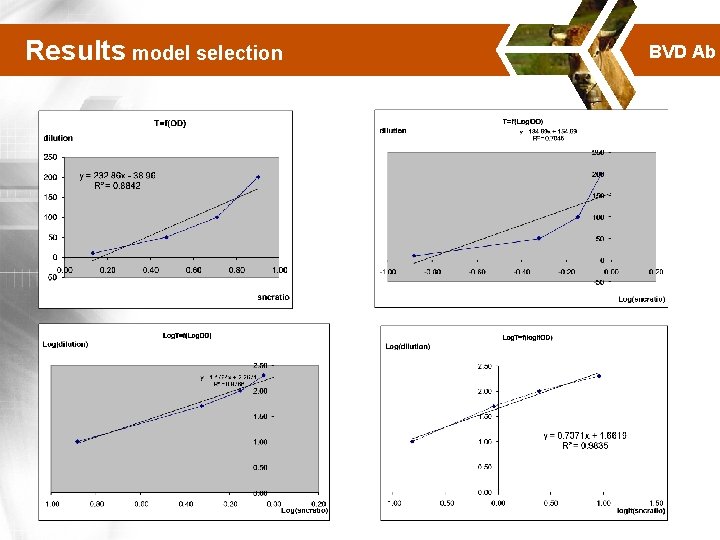
Results model selection BVD Ab

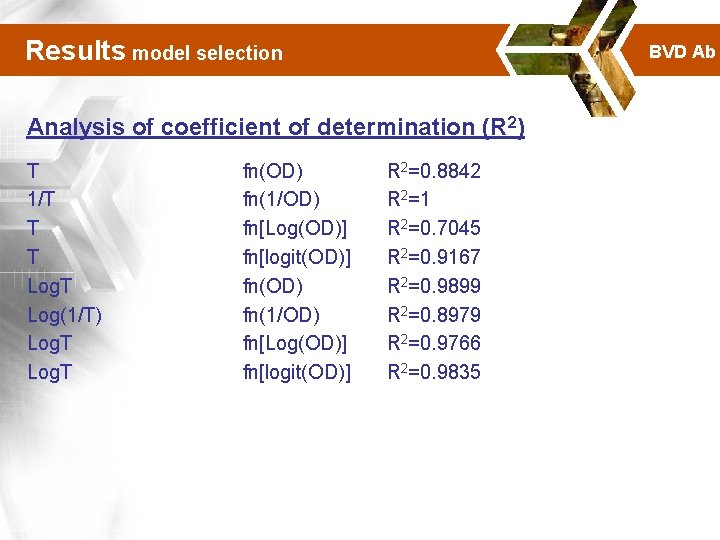
Results model selection BVD Ab Analysis of coefficient of determination (R 2) T 1/T T T Log(1/T) Log. T fn(OD) fn(1/OD) fn[Log(OD)] fn[logit(OD)] R 2=0. 8842 R 2=1 R 2=0. 7045 R 2=0. 9167 R 2=0. 9899 R 2=0. 8979 R 2=0. 9766 R 2=0. 9835

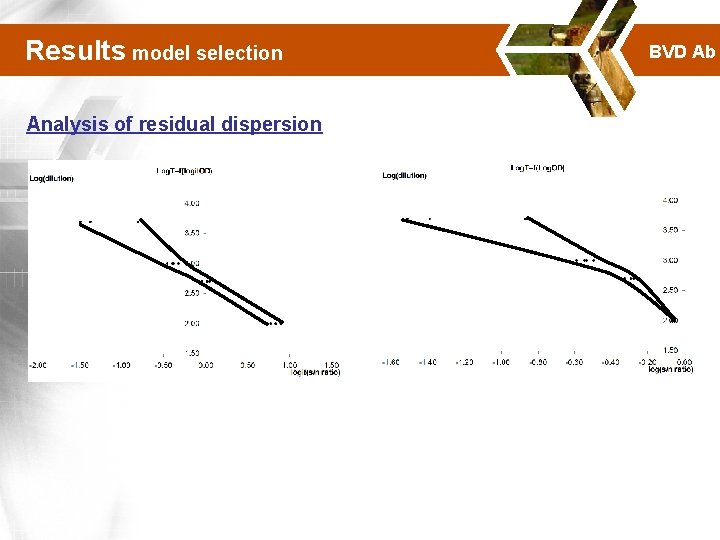
Results model selection Analysis of residual dispersion BVD Ab

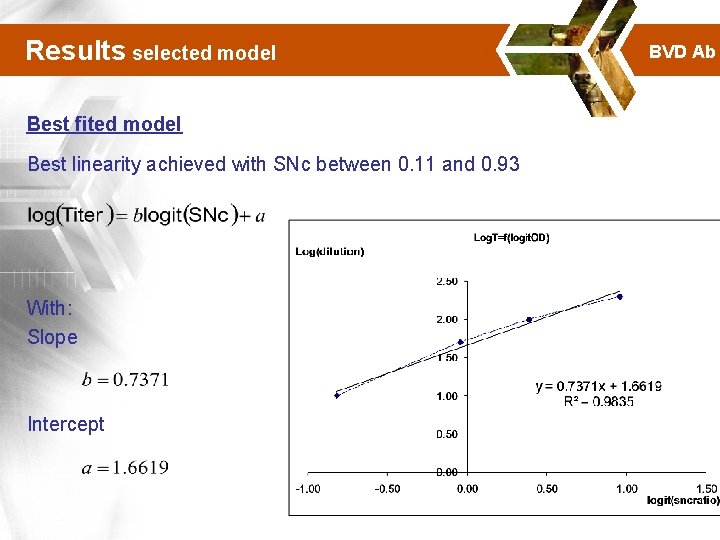
Results selected model Best fited model Best linearity achieved with SNc between 0. 11 and 0. 93 With: Slope Intercept BVD Ab

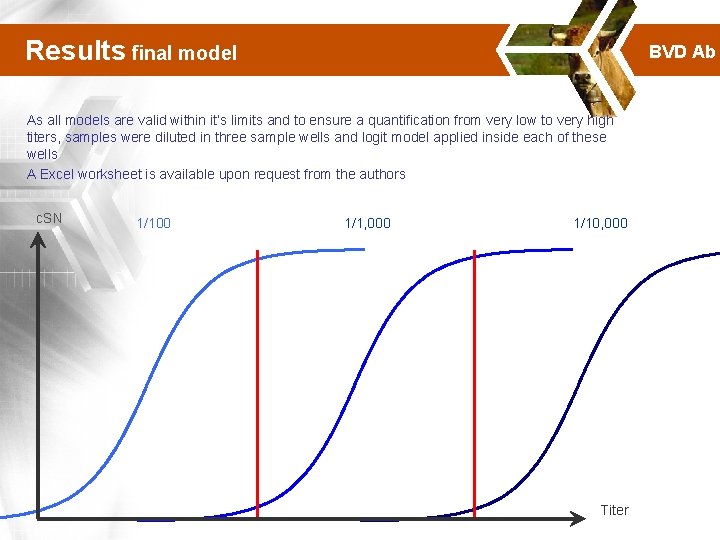
Results final model BVD Ab As all models are valid within it’s limits and to ensure a quantification from very low to very high titers, samples were diluted in three sample wells and logit model applied inside each of these wells A Excel worksheet is available upon request from the authors c. SN 1/100 1/1, 000 1/10, 000 Titer

Discussion/Conclusion Innovative quantitative method for specific detection of anti-NS 3 (p 80) antibodies against BVDV using latest quantitative model from human medicine/biostatistics Excellent linearity Quantitative method for a specific antibody subpopulation using a blocking ELISA This standardized quantitative test is a tool that will lead to a breakthrough in the understanding of the BVDV epidemiology by monitoring antibodies populations and be utilized to assess BVDV control measures. BVD Ab

Acknowledgments BVD Ab This project was funded through the Kansas State University College of Veterinary Medicine and the Beef Cattle Institute in conjunction with Synbiotics Corporation