Development anatomy of the respiratory organ 3 Organ























- Slides: 76

Development anatomy of the respiratory organ


3

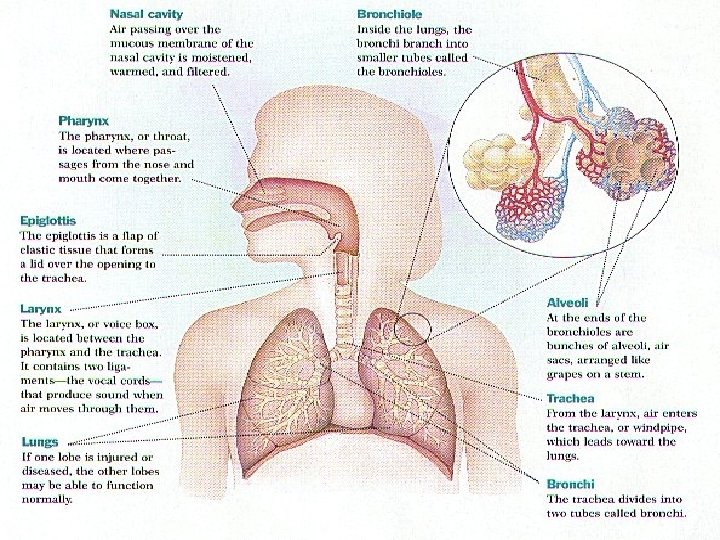
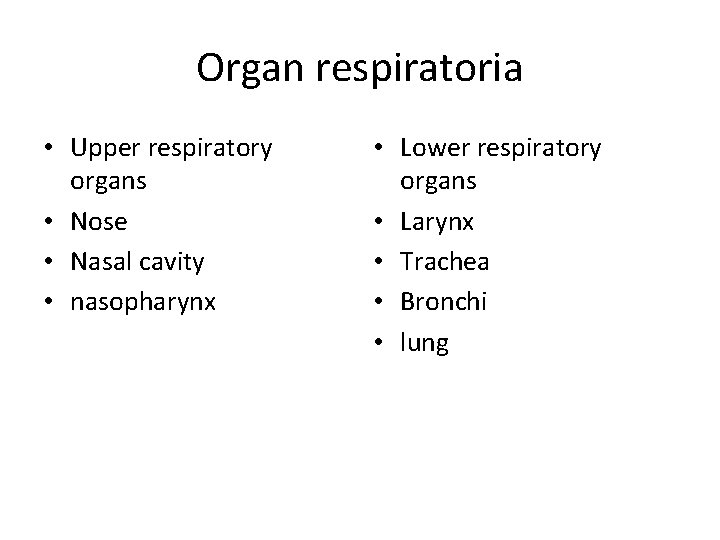
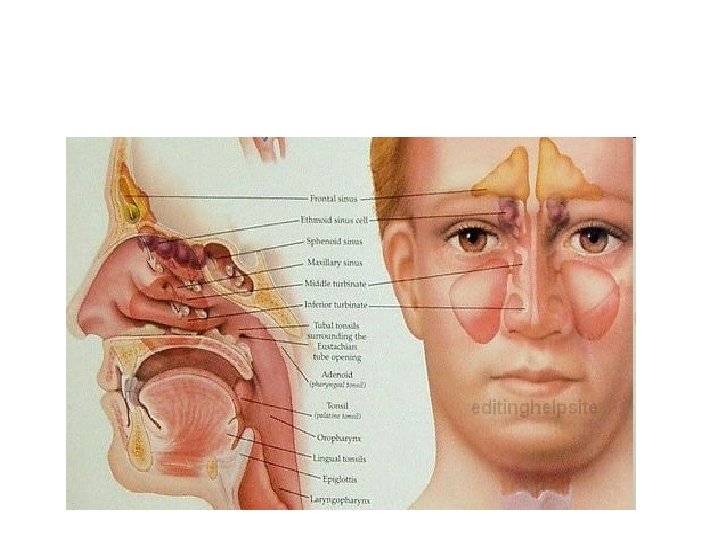
Organ respiratoria • Upper respiratory organs • Nose • Nasal cavity • nasopharynx • Lower respiratory organs • Larynx • Trachea • Bronchi • lung


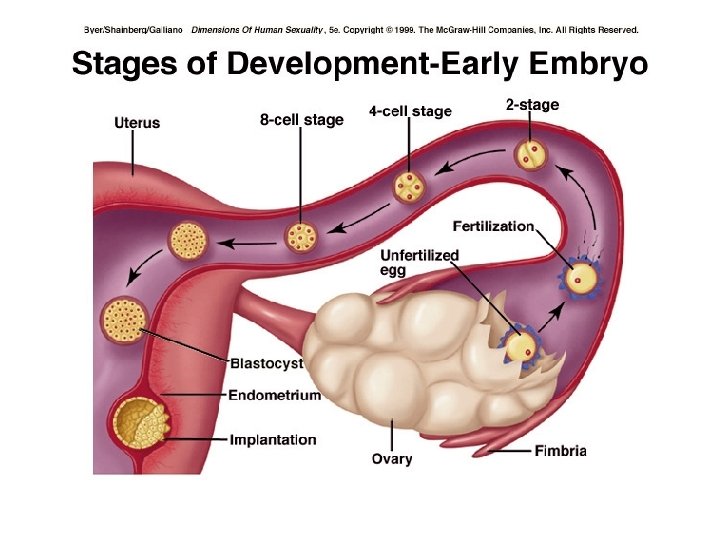
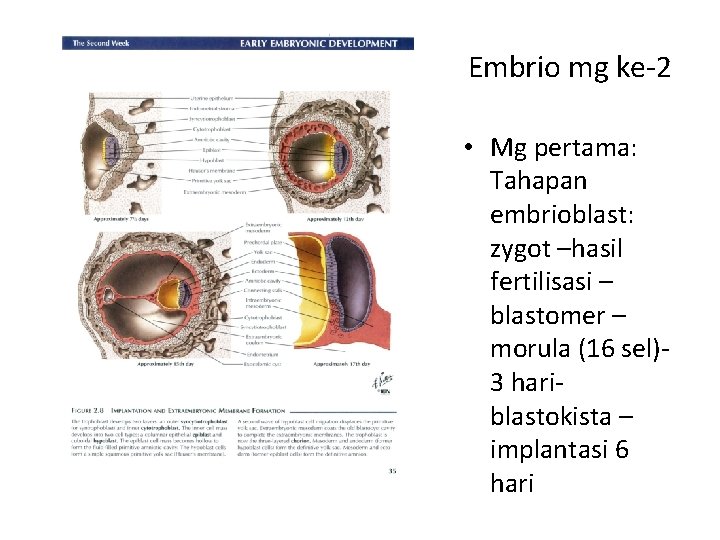
Embrio mg ke-2 • Mg pertama: Tahapan embrioblast: zygot –hasil fertilisasi – blastomer – morula (16 sel)- 3 hari- blastokista – implantasi 6 hari

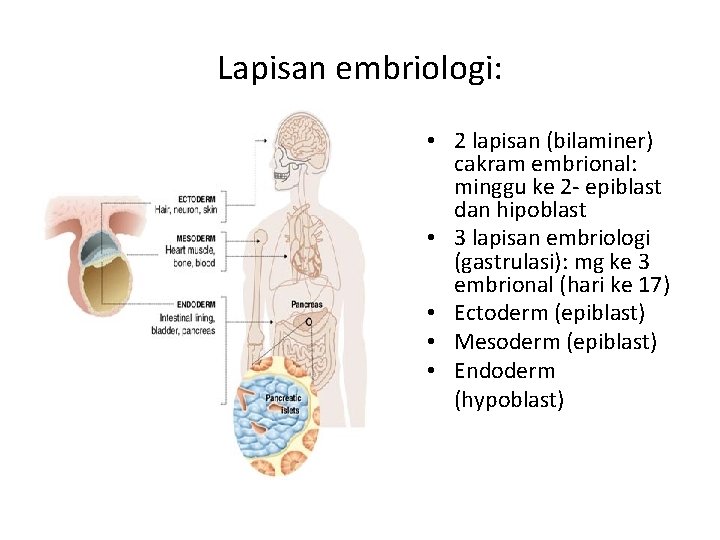
Lapisan embriologi: • 2 lapisan (bilaminer) cakram embrional: minggu ke 2 - epiblast dan hipoblast • 3 lapisan embriologi (gastrulasi): mg ke 3 embrional (hari ke 17) • Ectoderm (epiblast) • Mesoderm (epiblast) • Endoderm (hypoblast)

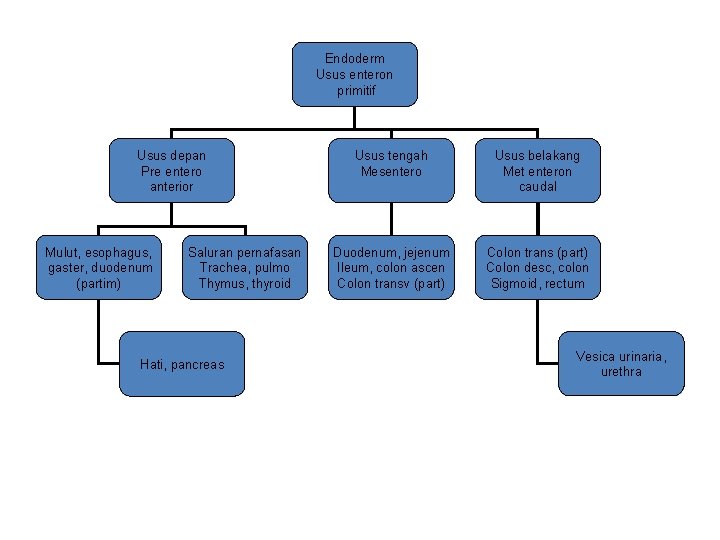
Endoderm Usus enteron primitif Usus depan Pre entero anterior Mulut, esophagus, gaster, duodenum (partim) Saluran pernafasan Trachea, pulmo Thymus, thyroid Hati, pancreas Usus tengah Mesentero Usus belakang Met enteron caudal Duodenum, jejenum Ileum, colon ascen Colon transv (part) Colon trans (part) Colon desc, colon Sigmoid, rectum Vesica urinaria, urethra

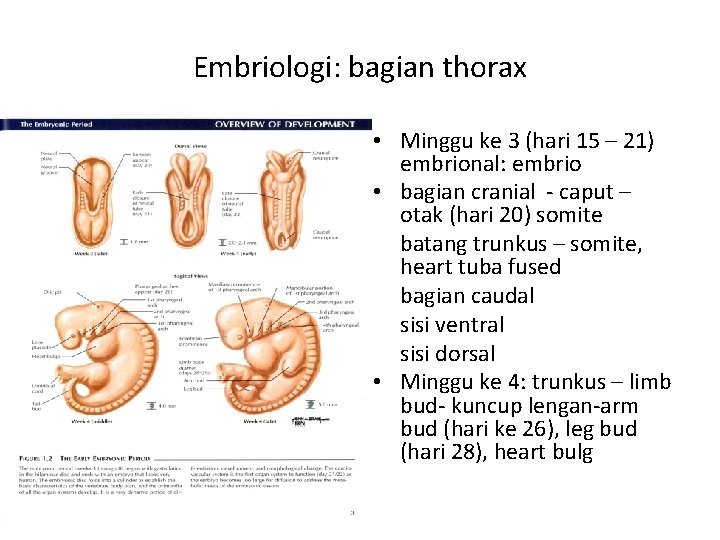
Embriologi: bagian thorax • Minggu ke 3 (hari 15 – 21) embrional: embrio • bagian cranial - caput – otak (hari 20) somite batang trunkus – somite, heart tuba fused bagian caudal sisi ventral sisi dorsal • Minggu ke 4: trunkus – limb bud- kuncup lengan-arm bud (hari ke 26), leg bud (hari 28), heart bulg

somite


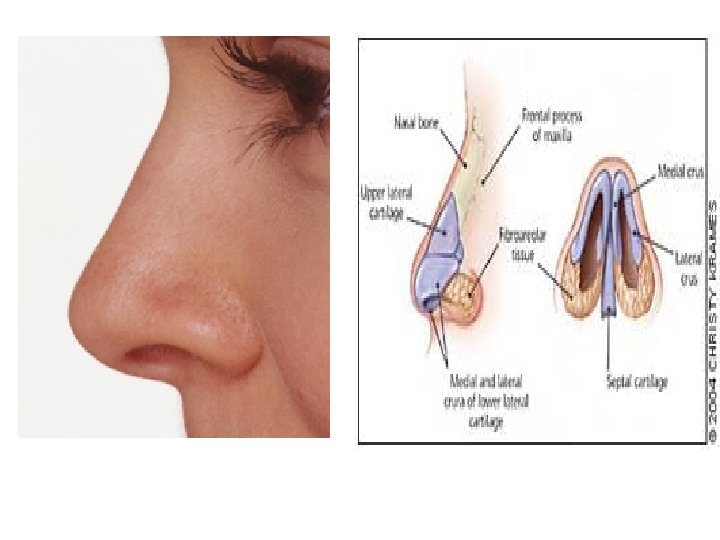
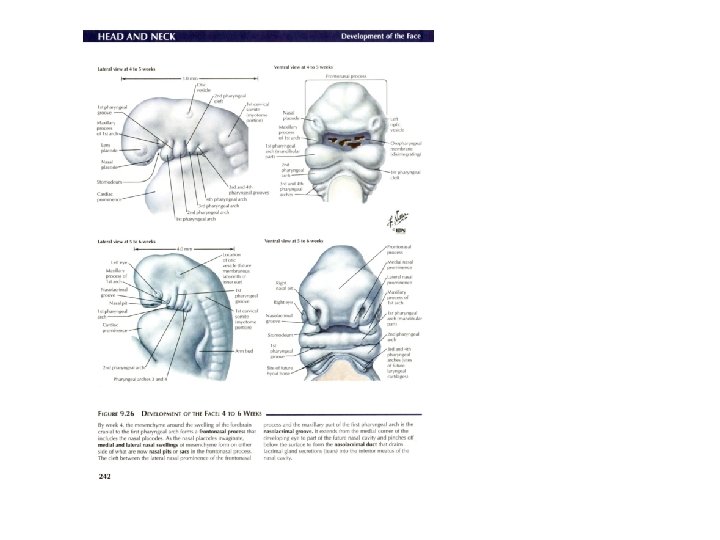
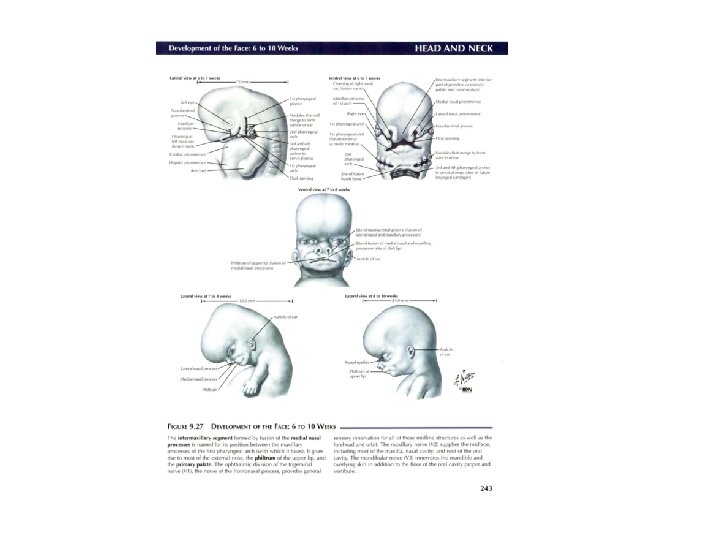
Development of face: nose • Nasal placodes and medial and lateral nasal processes • Nasal cofactor placode: two ectodermal elevation on each side of median plane of frontonasal process –and surface depression, and the edges become nasal process, the lateral more prominent, forms alae of nose • Medial process merge each other, as result growing of maxillar eminence (maxillar process), become middle part of upper lip, upper jaw, primary palate • Remains of maxillar process become cheek • Mandibular process: lower lip and lower jaw • Formation eyes: from lens placode; Formation external ear, from ectodermal cleft from series mesodermal thickening (pinna)



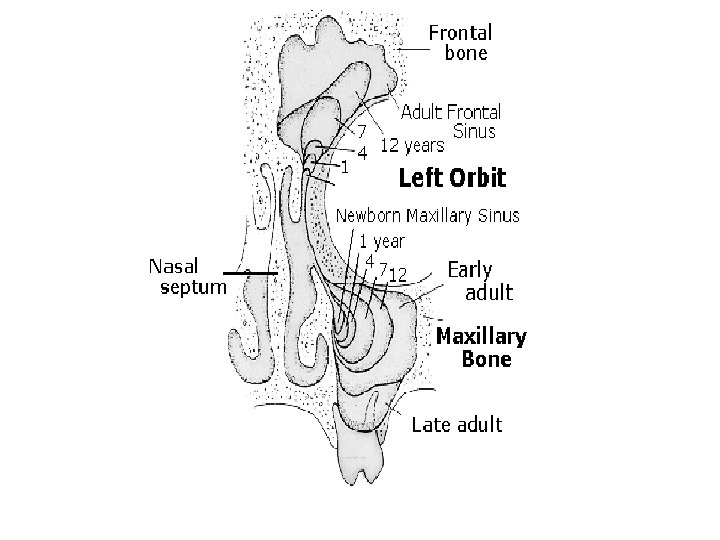
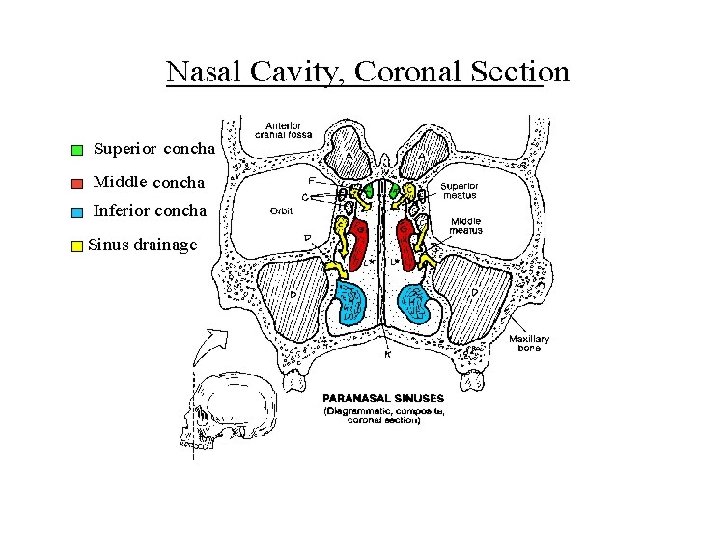
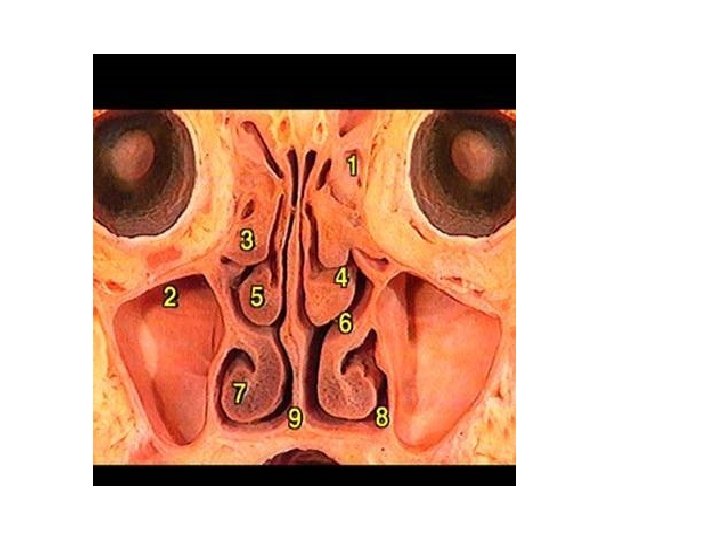
Development of nasal cavity • Formation of nasal septum, the nasal sacs, separated each other by the with • Formation of nasal pit, after intervening part of primitive palate formed by frontonasal process fusion lateral and medial • Secondary palate, separates nasal processes, created nasal cavities from mouth partition between nasal pits cavity and stomatodeum • Formation structures of • Formation of nasal lateral wall: lateral nasal sacs, nasal pits deepen and process, nasal conchae, enlarge dorsally, caudally olfactory epithelium form nasal sacs (ectodermal thickening) • Posterior part of nasal sacs, • Paranasal sinus: xaxillary and separated by buconasal sphenoid formed end of membrane, and soon fetal life, other formed after disappears, forming birth posterior nares




• pharynx: cephalic (prelaryngeal) of foregut (from buccopharyngeal membrane to tracheobroncheal diverticulum) • Definitive pharynx: primitive pharynx after formed branchial apparatus (palate & mouth) • Nasopharynx: communicate ventrally, forms cranial part of stomatodeum after formation palate • Nasopharynx: from stomatodeum after ruptured buccopharyneal membrane • Laryngopharynx: tracheobronchial diverticulum

Anomaly of nasal cavity • Atresia of nasal cavity • Anomaly of nasal septum, deflected of nasal septum and absence of septum • Cleft palate (abnormal communication mouth and nasal cavity)


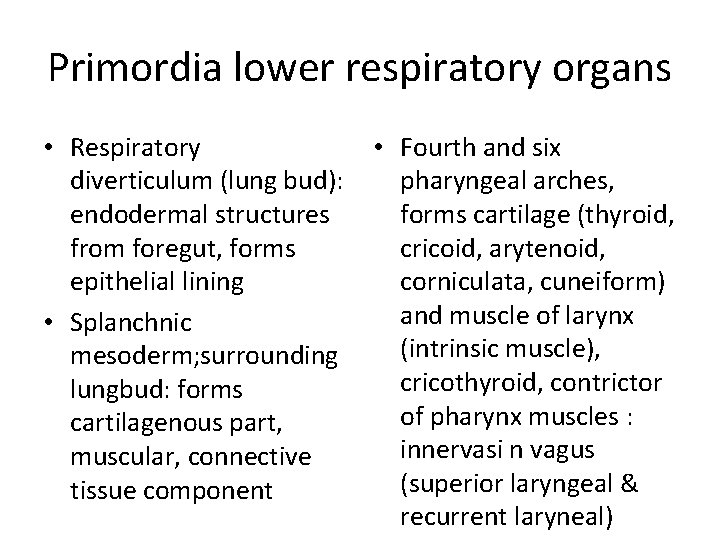
Primordia lower respiratory organs • Respiratory • Fourth and six diverticulum (lung bud): pharyngeal arches, endodermal structures forms cartilage (thyroid, from foregut, forms cricoid, arytenoid, epithelial lining corniculata, cuneiform) and muscle of larynx • Splanchnic (intrinsic muscle), mesoderm; surrounding cricothyroid, contrictor lungbud: forms of pharynx muscles : cartilagenous part, innervasi n vagus muscular, connective (superior laryngeal & tissue component recurrent laryneal)

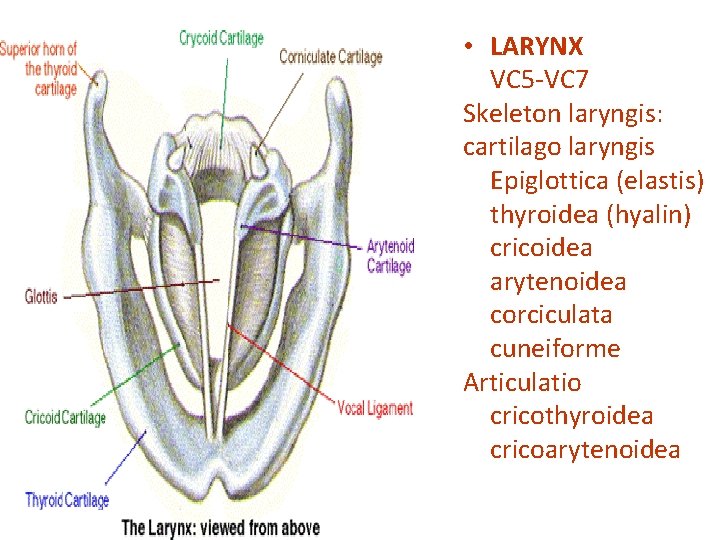
• LARYNX VC 5 -VC 7 Skeleton laryngis: cartilago laryngis Epiglottica (elastis) thyroidea (hyalin) cricoidea arytenoidea corciculata cuneiforme Articulatio cricothyroidea cricoarytenoidea


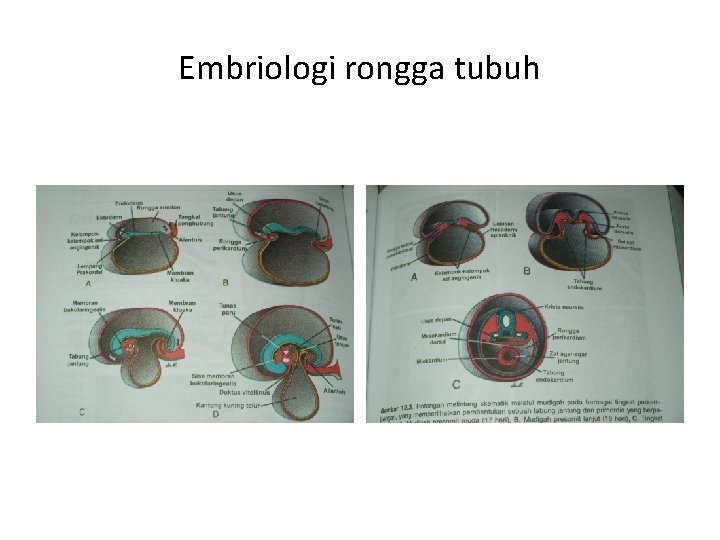
Embriologi rongga tubuh

Rongga rongga tubuh • Pembentukan selom intraembrional mg 3: sebelumnya – rongga amnion, selom ekstraembrional, kantong kuning telur • Akhir mg 3; mesoderm lateral – somatik & splanknik -- selom intraembrional (dada sampai panggul) • Lapisan somatik – lapisan parietal membran serosa – rongga peritoneum, pleura dan kantong jantung; Lapisan splanknik – lapisan visceral m. serosa nya – di dorsal sbg mesenterium dorsale, dan di ventral sbg mesenterium ventrale (di usus depan) krn ada septum transversum

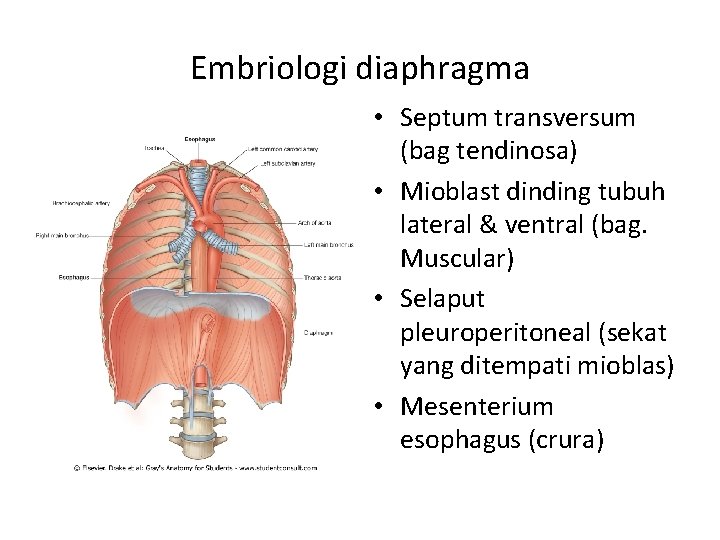
Diaphragma dan rongga dada • Septum transversum – sekat mesoderm : rongga dada – tangkai kantong kuning telur • Tidak memisahkan sempurna rongga dada & rongga perut • Lubang: saluran perikardioperitoneal di kiri kanan usus depan • Ada tunas pulmo, tumbuh cepat di dalam saluran perikardioperitoneal, lipatan pleuroperikardial – rigi menonjol – ruang dada primitif, pulmo meluas, rongga mesoderma dinding tubuh – dibelah 2 komponen: dinding dada definitif dan membrana pleuroperikardial • Jantung turun, sinus venosus, membrana tertarik keluar – rongga dada; rongga perikardium tetap & dua rongga pleura tetap

Rongga dada & perut serta diaphragma • Mg ke 7: rongga dada & perut tertutup oleh selaput pleuroperitoneal (: lipatan pleuroperitoneal), septum transversom, mesenterium esophagus--- mioblas dinding tubuh membentuk bagian otot diaphragma • Klinis : hernia diaphragmatica

Embriologi diaphragma • Septum transversum (bag tendinosa) • Mioblast dinding tubuh lateral & ventral (bag. Muscular) • Selaput pleuroperitoneal (sekat yang ditempati mioblas) • Mesenterium esophagus (crura)

Perkembangan bagian badan

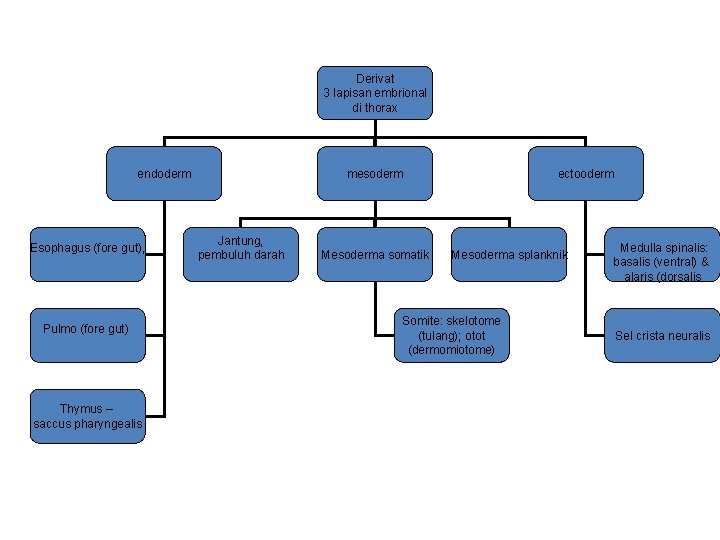
Derivat 3 lapisan embrional di thorax endoderm Esophagus (fore gut), Pulmo (fore gut) Thymus – saccus pharyngealis mesoderm Jantung, pembuluh darah Mesoderma somatik ectooderm Mesoderma splanknik Somite: skelotome (tulang); otot (dermomiotome) Medulla spinalis: basalis (ventral) & alaris (dorsalis Sel crista neuralis

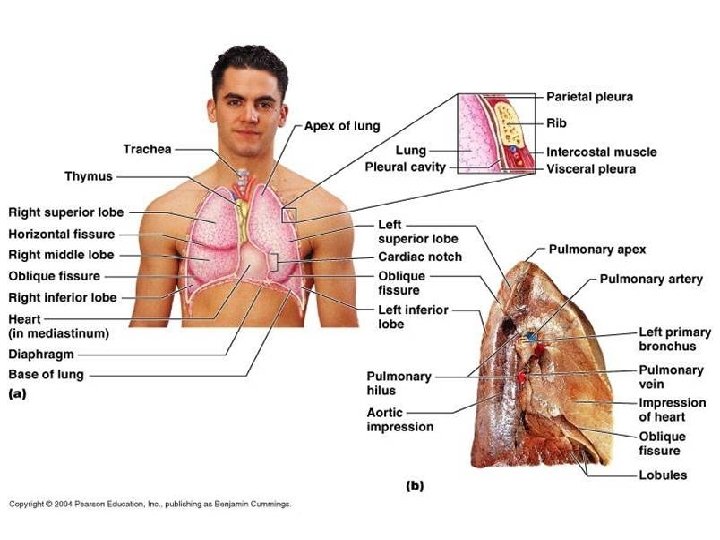
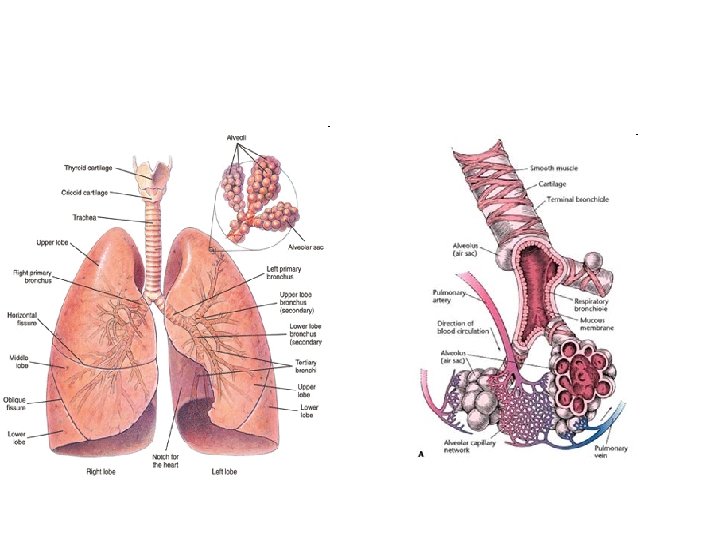
Embriologi trachea & pulmo • Embrio 4 mg: tunas pulmo (diverticulum respiratorium) – tonjolan keluar dari dinding ventral usus depan • Epitel – larynx, trachea, bronchus, epitel paru dari endoderm • Tulang rawan, otot polos – dari mesoderma splanknik sekeliling usus depan • Rigi esophagotrachealis – septum – trpisah: esophagus & trachea dan tunas pulmo

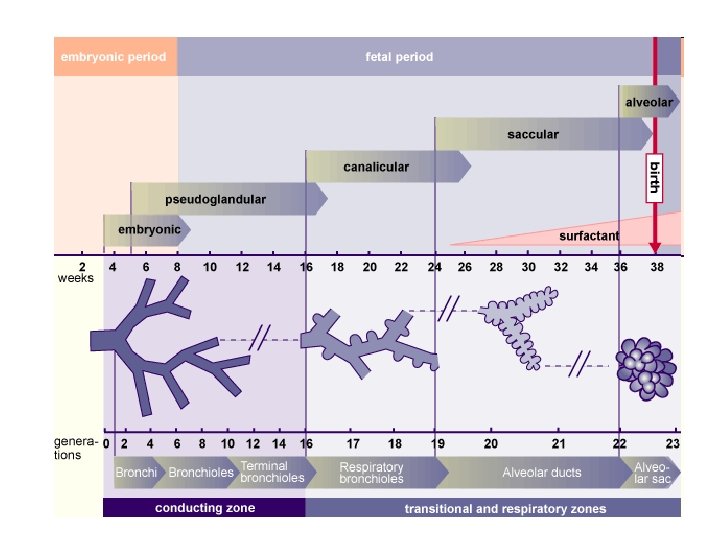
Phases of lung development • Embryonic phase • Pseudoglandular phase • Canalicular phase • Saccular phase • Alveolar phase • Classification in the adult lung



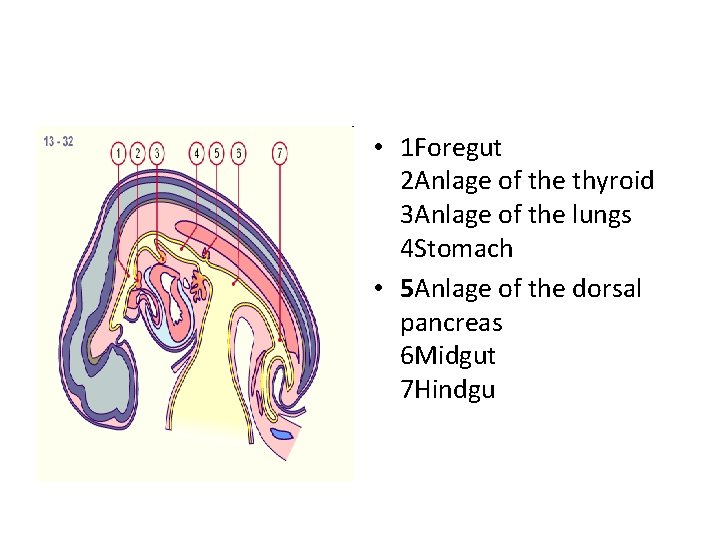
• 1 Foregut 2 Anlage of the thyroid 3 Anlage of the lungs 4 Stomach • 5 Anlage of the dorsal pancreas 6 Midgut 7 Hindgu

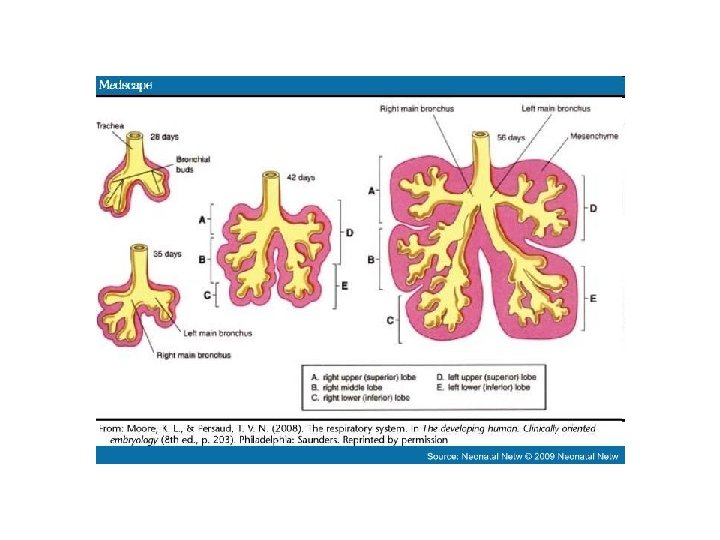
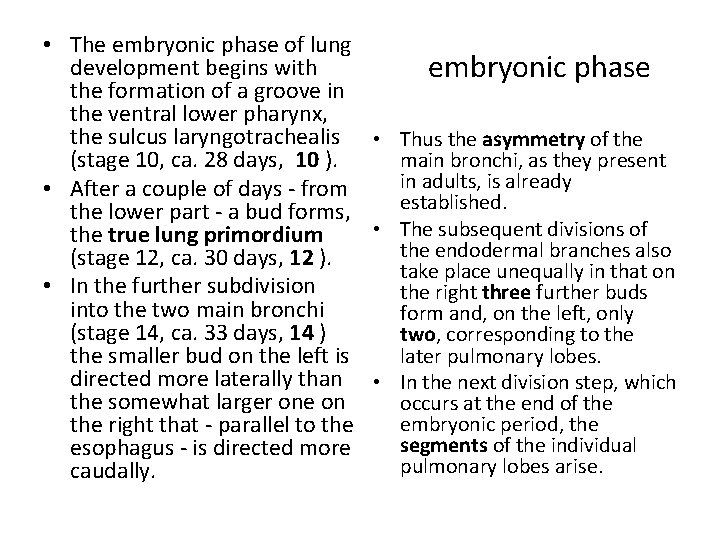
• The embryonic phase of lung development begins with embryonic phase the formation of a groove in the ventral lower pharynx, the sulcus laryngotrachealis • Thus the asymmetry of the (stage 10, ca. 28 days, 10 ). main bronchi, as they present in adults, is already • After a couple of days - from established. the lower part - a bud forms, • The subsequent divisions of the true lung primordium the endodermal branches also (stage 12, ca. 30 days, 12 ). take place unequally in that on • In the further subdivision the right three further buds into the two main bronchi form and, on the left, only (stage 14, ca. 33 days, 14 ) two, corresponding to the later pulmonary lobes. the smaller bud on the left is directed more laterally than • In the next division step, which the somewhat larger one on occurs at the end of the embryonic period, the right that - parallel to the segments of the individual esophagus - is directed more pulmonary lobes arise. caudally.

• at the end of the embryonic period the first segments appear in the five (three right and two left) lobes of the lungs. • With their distended ends the lungs resemble an exocrine gland. • At this time the pulmonary vessels have formed themselves. • The pulmonary circulation system (smaller circulation system) is formed out of the 6 th pharyngeal arch artery. • These develop somewhat differently than the other 4 aortic arches in that first a vessel plexus forms around the lung anlage, originating from the aortic sac. • The true 6 th aortic arch is only then formed after vessels - also from the dorsal aorta - grow into this plexus and thus a connection between the truncus pulmonalis and dorsal aorta has arisen.

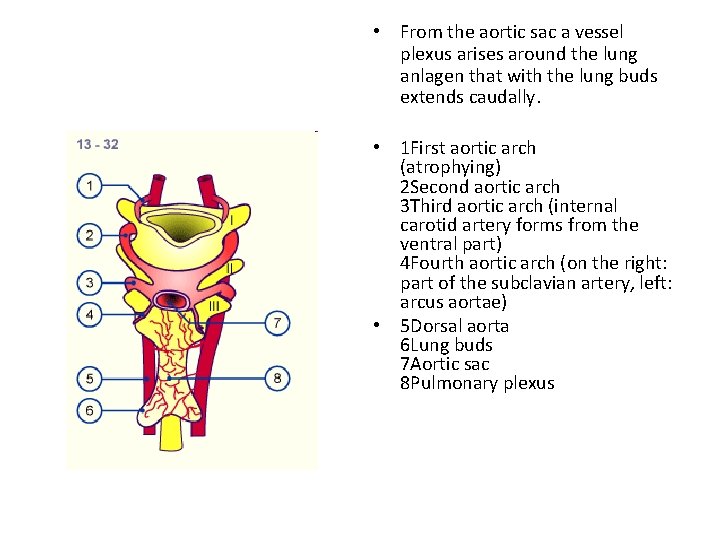
• From the aortic sac a vessel plexus arises around the lung anlagen that with the lung buds extends caudally. • 1 First aortic arch (atrophying) 2 Second aortic arch 3 Third aortic arch (internal carotid artery forms from the ventral part) 4 Fourth aortic arch (on the right: part of the subclavian artery, left: arcus aortae) • 5 Dorsal aorta 6 Lung buds 7 Aortic sac 8 Pulmonary plexus

• At this stage the lungs resemble the development of a tubuloacinous gland. • According to the classical view, the entire air-conducting bronchial tree up to the terminal bronchioli are set down in this phase (16 generations). • Recent morphometric studies (3) have shown that with the end of the pseudoglandular phase 20 generations are partially present in the lungs, which means that at this point in time the respiratory ducts have already been formed. The primordial system of passages, the air-conducting bronchial tree, is initially coated by cubic epithelium Pseudoglandular phase • These are the precursor cells of the ciliated epithelium and of the secretory cells. In humans, the first ciliated epithelial cells can be found in the 13 th week of pregnancy (7). In the respiratory part the first typically lung-specific cells, connected to the terminal bronchioli, appear: the type II pneumocytes (alveolar cells) (3). The developing bronchopulmonary epithelium begins to produce amniotic fluid, which is also found in the lungs up to the time of birth.

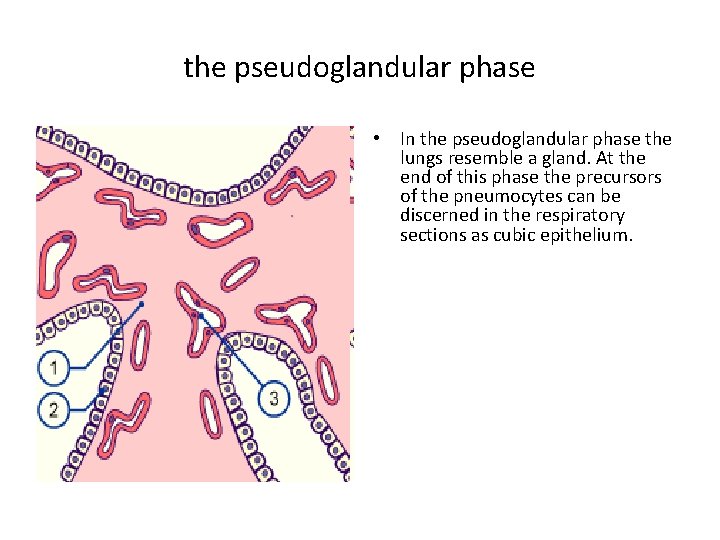
the pseudoglandular phase • In the pseudoglandular phase the lungs resemble a gland. At the end of this phase the precursors of the pneumocytes can be discerned in the respiratory sections as cubic epithelium.

• Relatively early in the development of the lungs, endocrine-active cells (Kultschitsky cells) appear that produce bombesin and serotonin. • In contrast to the precursors of the pneumocytes, which originate from the endoderm, they stem from the neural crest (neuroectoderm). • Via paracrine mechanisms bombesin probably plays a decisive role for lung development in that mainly the type II pneumocytes proliferate. (1) • The differentiation of the lungs takes place in a centrifugal direction. In the central, airconducting portions of the lungs the epithelium begins to differentiate into cilia-carrying cells and goblet cells. • After the 10 th week cartilage and smooth muscle cells as well as bronchial glands can be found in the wall of the bronchi. • The peripheral sections partially retain - until far beyond the pseudoglandular phase - cubic epithelium that is still little differentiated

• This is important for a further proliferation of the bronchial tree into the surrounding mesenchymal tissue. • f one begins, roughly estimated, with a number of 15'000 terminal bronchioli (8) per lung in adults and thereby ca. 15, 000 acini and with a theoretical assumption of a dichotomous division of the pulmonary branches, one has the result that this stage is attained after little fewer than 2 14 generations. • In the late pseudoglandular stage one finds, however, far more than 15'000 end pieces. Thus the lung end pieces at this stage already represent the respiratory portions of the lungs.

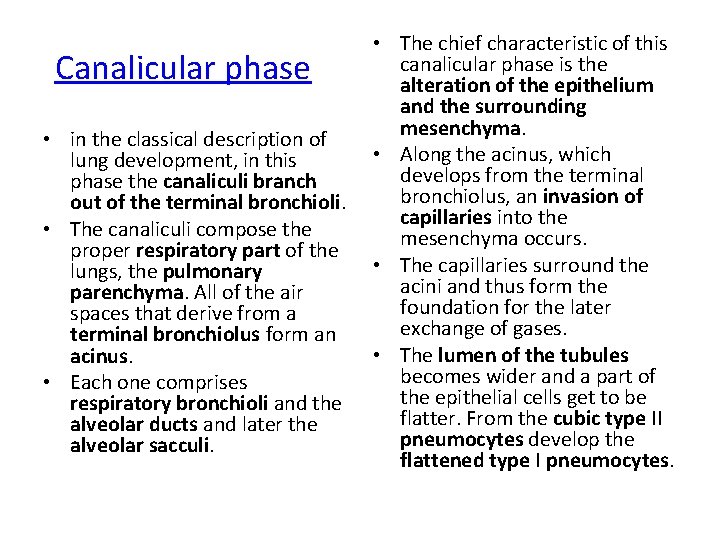
• The chief characteristic of this canalicular phase is the alteration of the epithelium and the surrounding mesenchyma. • in the classical description of • Along the acinus, which lung development, in this develops from the terminal phase the canaliculi branch bronchiolus, an invasion of out of the terminal bronchioli. capillaries into the • The canaliculi compose the mesenchyma occurs. proper respiratory part of the • The capillaries surround the lungs, the pulmonary acini and thus form the parenchyma. All of the air foundation for the later spaces that derive from a exchange of gases. terminal bronchiolus form an • The lumen of the tubules acinus. becomes wider and a part of • Each one comprises the epithelial cells get to be respiratory bronchioli and the flatter. From the cubic type II alveolar ducts and later the pneumocytes develop the alveolar sacculi. flattened type I pneumocytes. Canalicular phase

• A sufficient differentiation of the type II pneumocytes into the type I pneumocytes and the proliferation of the capillaries into the mesenchyma marks an important step towards the fetus being able to survive outside the uterus after roughly the 24 th week of pregnancy.

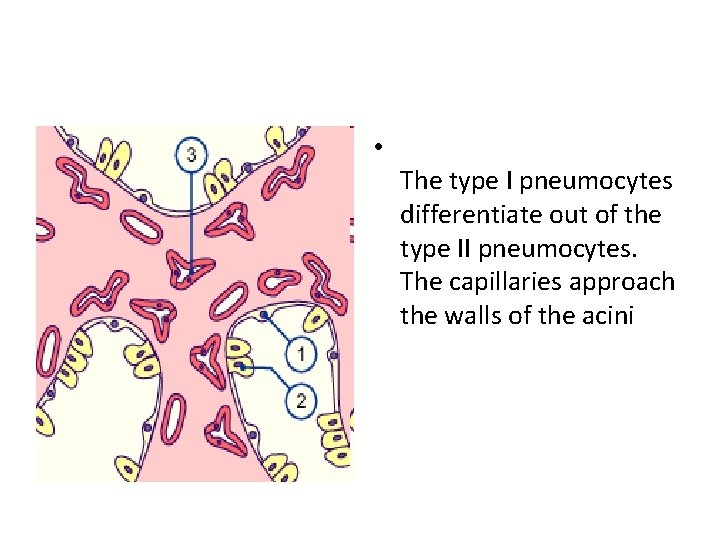
• The type I pneumocytes differentiate out of the type II pneumocytes. The capillaries approach the walls of the acini

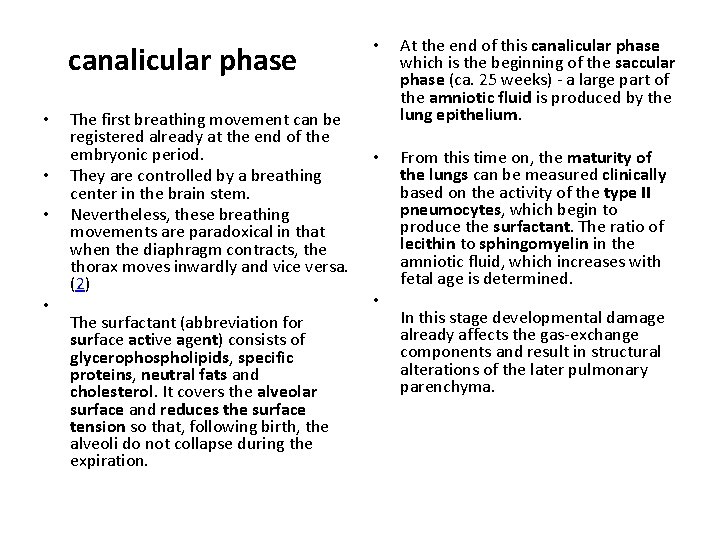
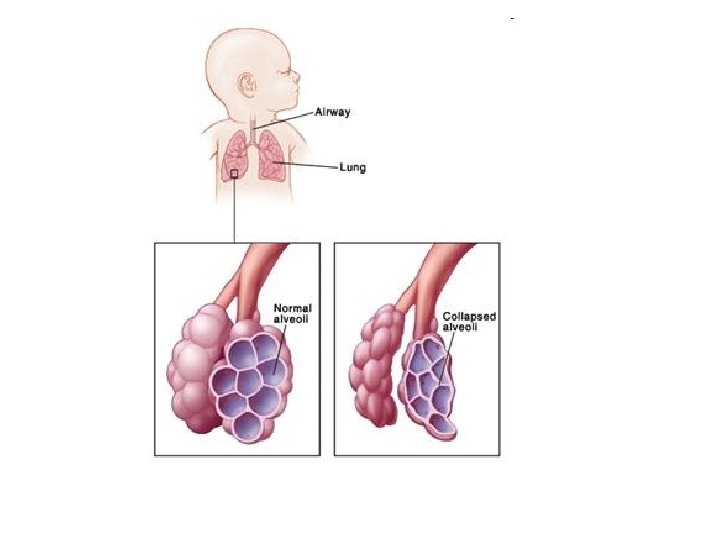
canalicular phase • • The first breathing movement can be registered already at the end of the embryonic period. They are controlled by a breathing center in the brain stem. Nevertheless, these breathing movements are paradoxical in that when the diaphragm contracts, the thorax moves inwardly and vice versa. (2) The surfactant (abbreviation for surface active agent) consists of glycerophospholipids, specific proteins, neutral fats and cholesterol. It covers the alveolar surface and reduces the surface tension so that, following birth, the alveoli do not collapse during the expiration. • At the end of this canalicular phase which is the beginning of the saccular phase (ca. 25 weeks) - a large part of the amniotic fluid is produced by the lung epithelium. • From this time on, the maturity of the lungs can be measured clinically based on the activity of the type II pneumocytes, which begin to produce the surfactant. The ratio of lecithin to sphingomyelin in the amniotic fluid, which increases with fetal age is determined. • In this stage developmental damage already affects the gas-exchange components and result in structural alterations of the later pulmonary parenchyma.

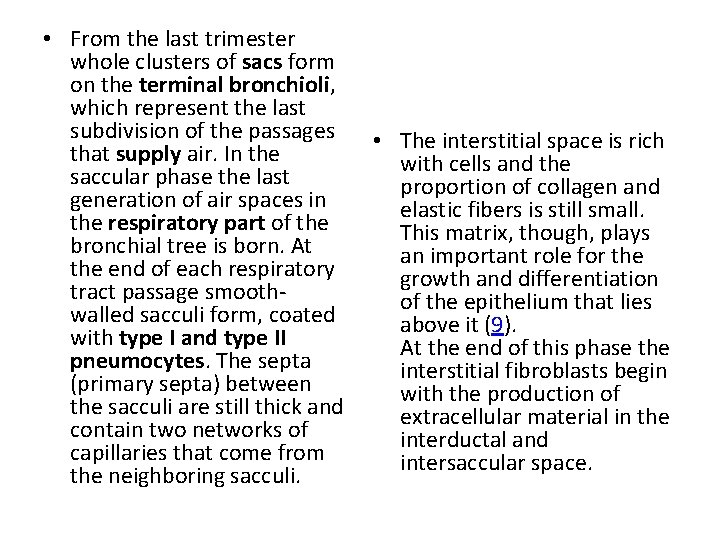
• From the last trimester whole clusters of sacs form on the terminal bronchioli, which represent the last subdivision of the passages • The interstitial space is rich that supply air. In the with cells and the saccular phase the last proportion of collagen and generation of air spaces in elastic fibers is still small. the respiratory part of the This matrix, though, plays bronchial tree is born. At an important role for the end of each respiratory growth and differentiation tract passage smoothof the epithelium that lies walled sacculi form, coated above it (9). with type I and type II At the end of this phase the pneumocytes. The septa interstitial fibroblasts begin (primary septa) between with the production of the sacculi are still thick and extracellular material in the contain two networks of interductal and capillaries that come from intersaccular space. the neighboring sacculi.

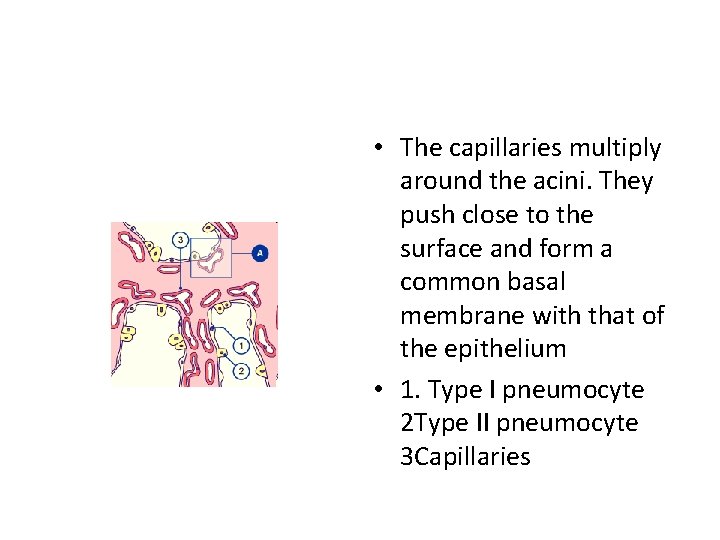
• The capillaries multiply around the acini. They push close to the surface and form a common basal membrane with that of the epithelium • 1. Type I pneumocyte 2 Type II pneumocyte 3 Capillaries

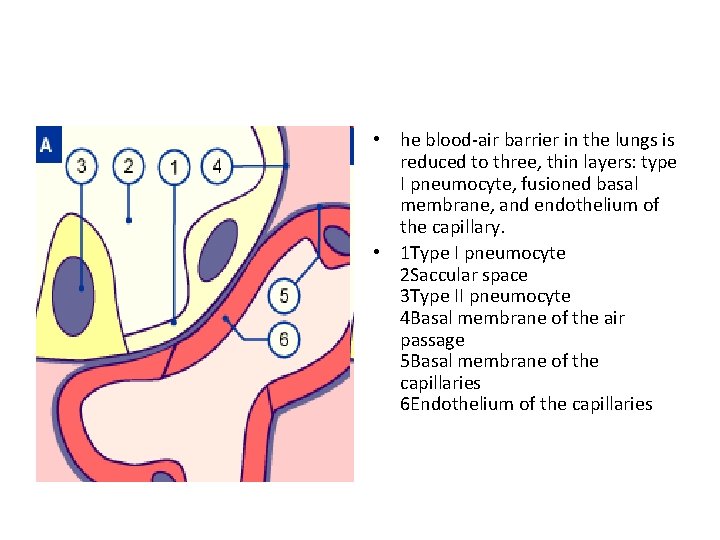
• he blood-air barrier in the lungs is reduced to three, thin layers: type I pneumocyte, fusioned basal membrane, and endothelium of the capillary. • 1 Type I pneumocyte 2 Saccular space 3 Type II pneumocyte 4 Basal membrane of the air passage 5 Basal membrane of the capillaries 6 Endothelium of the capillaries

the saccular phase, • At birth, i. e. , at the end of the saccular phase, all generations of the conducting and respiratory branches have been generated. The sacculi are thin, smooth-walled sacks and correspond to the later alveolar sacculi.

• Depending on the author, the alveolar phase begins at varying times. Probably in the last few weeks of • Between them lies the pregnancy, new parenchyma, composed sacculi and, from them, of a double layer of the first alveoli form. capillaries, that forms the Thus, at birth, ca. 1/3 of primary septa between the roughly 300 million the alveolar sacculi. alveoli should be fully developed. The alveoli, though, are only present in their beginning forms. alveolar phase

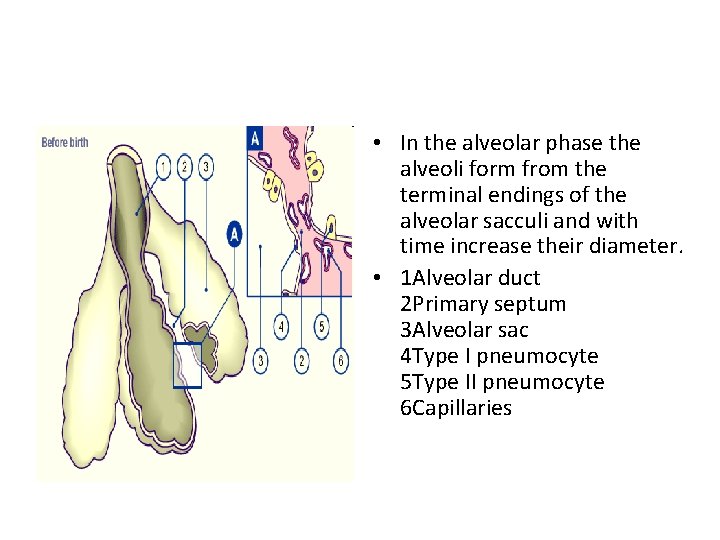
• In the alveolar phase the alveoli form from the terminal endings of the alveolar sacculi and with time increase their diameter. • 1 Alveolar duct 2 Primary septum 3 Alveolar sac 4 Type I pneumocyte 5 Type II pneumocyte 6 Capillaries

• Already before birth these alveolar sacculi get to be increasingly complex structurally. • Thereby, a large number of small protrusions form along the primary septa. Soon, these become larger and subdivide the sacculi into smaller subunits, the alveoli, which are delimited by secondary septa. • Ultrastructural investigations show that overall where such alveoli appear, they are surrounded by elastic fibers that form the interstitial septa between two capillary nets. In the first 6 months, their number increases massively. This "alveolarization" and therewith the formation of secondary septa should - to a limited extent still - continue up to the first year and a half of life.

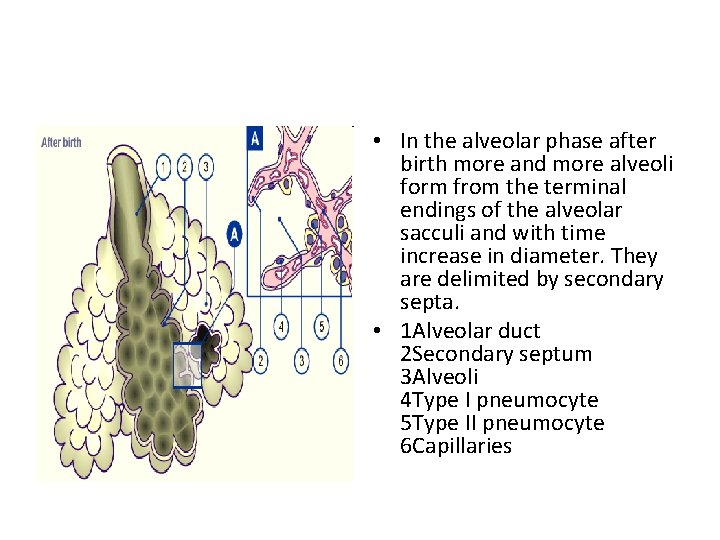
• In the alveolar phase after birth more and more alveoli form from the terminal endings of the alveolar sacculi and with time increase in diameter. They are delimited by secondary septa. • 1 Alveolar duct 2 Secondary septum 3 Alveoli 4 Type I pneumocyte 5 Type II pneumocyte 6 Capillaries



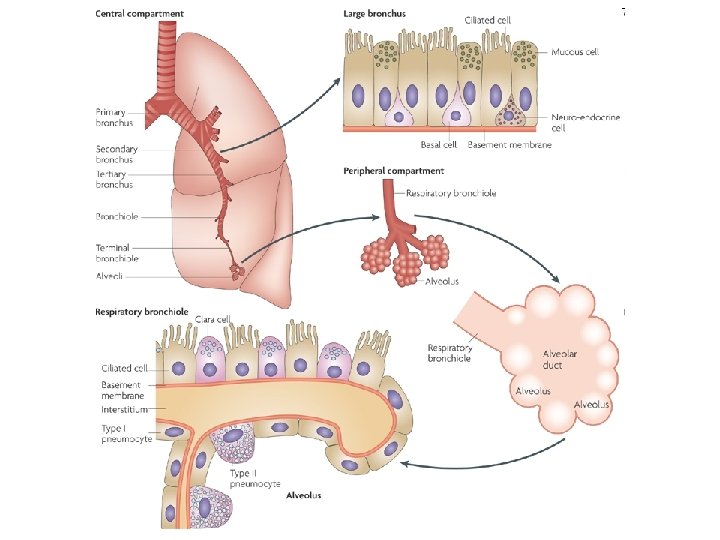
• In the adult lung one • As soon as cartilage and distinguishes between glands are no longer conducting and present, bronchioli are respiratory zones. involved. In the conducting zone, all branches of the bronchial tree, the walls of which contain cartilage tissue and seromucous glands, are bronchi.

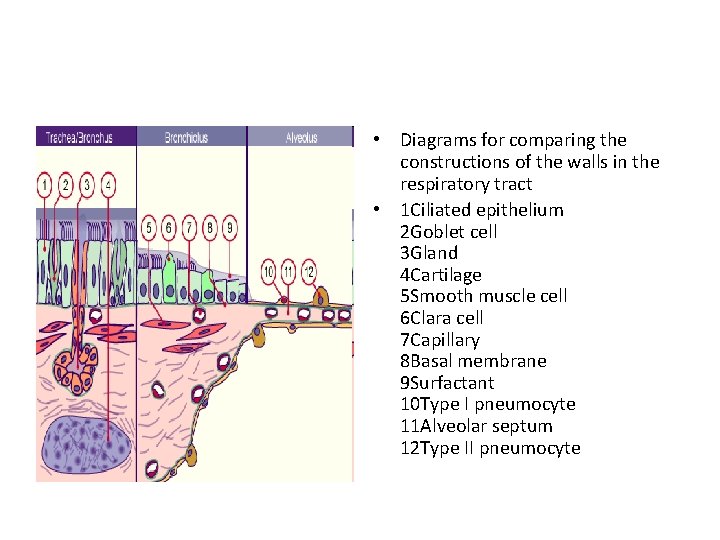
• Diagrams for comparing the constructions of the walls in the respiratory tract • 1 Ciliated epithelium 2 Goblet cell 3 Gland 4 Cartilage 5 Smooth muscle cell 6 Clara cell 7 Capillary 8 Basal membrane 9 Surfactant 10 Type I pneumocyte 11 Alveolar septum 12 Type II pneumocyte

• • • According to their function the respiratory tract passages are divided into conducting and respiratory zones: Conducting zone = 16 generations Segmental bronchi are continued by several generations of Intersegmental bronchi (up to ca. 1 mm diameter). After these follow the Bronchioli (< 1 mm diameter) that after several divisions go over into Terminal bronchioli (ca. 0. 4 mm diameter). They subdivide numerous times and represent the end stretch of the purely conductive respiratory tract. The measurements come from histological findings. • Histological image of respiratory epithelium. Respiratory zone = 7 generations • Out of the terminal bronchioli several generation of • Respiratory bronchioli (= 3 generations) proceed. From them follow several generations of • Alveolar ducts (= 3 generations) that in • Alveolar sacculi (last generation = 23 rd generation) end


• For the branching out of ever new lung buds an interaction between the respiratory endodermal epithelium and the • These molecules are found surrounding pulmonary around the passages and in the forks of the bronchial tree. mesenchyma is primarily They are responsible for the responsible. Mainly the stabilization of the already epidermal growth factor formed structures - these are (EGF) and the extracellular not present in the regions of form of the transforming the newly formed branches. growth factors (TGF-b) Epimorphine, a further appear to be important for protein, appears to promote lung development. the formation of epithelial In addition, one finds passages. If epimorphine is specific extracellular matrix blocked by antibodies, the components like collagen of epithelium that lies above it can not form itself into tubes types I and III, as well as and remains unorganized proteoglycan and the fibronectin and syndecan glycoproteins. . (

anomaly • Laryngeal atresia • Fistula tracheoesophageal • Tracheal stenosis, atresia • Agenesis trachea • Agenesis of lung • Hyaline membrane disease/respiratory distress syndrome


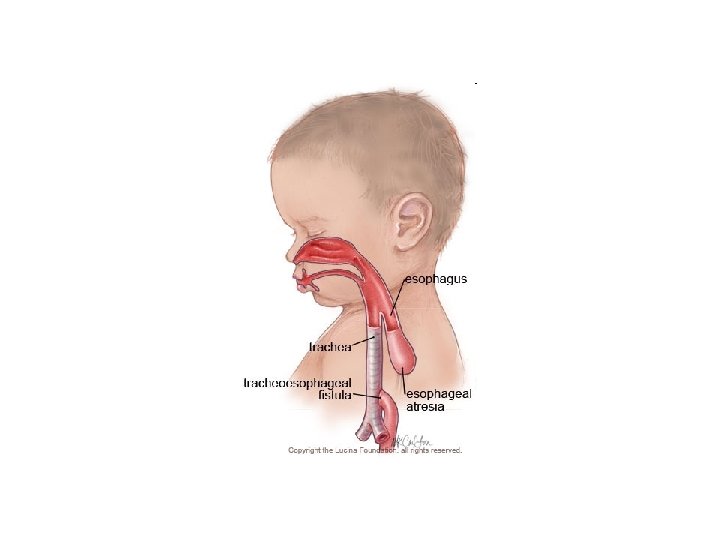
• Tracheoesophageal • Normally these two fistula tubes do not connect A fistula is a tube. The but when a baby has a esophagus is a tube that tracheoesophageal goes to the stomach fistula, there is a tube and the trachea is a connecting the two. tube that goes to the This can cause problems lungs. with feeding and even breathing in newborns and needs to be corrected.

• Oesophageal Atresia and/or Tracheoesophageal Fistula • Embryology Smith's theory: The trachea and esophagus initially begin as a single tube. The lateral esophageal grooves are formed as the dorsal esophagus is separated from the ventral trachea. Should the septation process continue distally, esophageal atresia would result. Grunewald's theory: Elongation of the trachea is rapid in a caudal direction. • When there is a fistula producing fixation of esophagus to trachea, the dorsal wall of the esophagus is drawn forward and downward to be incorporated into the trachea. Atresia of the esophagus results because of the fistula. Bronchogenic theory: The esophagus does not develop at all distally. Rather, a third "bronchus" develops in the primordial lung bud and grows inferiorly to attach to the stomach.

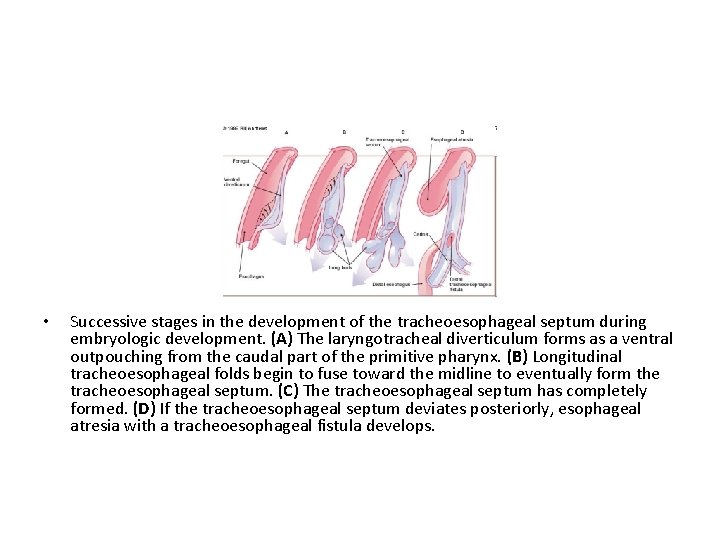
• The esophagus and trachea derive from the primitive foregut. During the fourth and fifth weeks of • This septum divides the embryologic development, the foregut into a ventral trachea forms as a portion, the laryngotracheal ventral diverticulum tube and a dorsal portion from the primitive (the esophagus). pharynx (caudal part of Esophageal atresia results if the foregut), 5 as the tracheoesophageal illustrated in Figure 3. A septum is deviated tracheoesophageal septum develops at the posteriorly. This deviation site where the causes incomplete longitudinal separation of the esophagus tracheoesophageal from the laryngotracheal folds fuse together. . tube and results in a concurrent tracheoesophageal fistula

• Successive stages in the development of the tracheoesophageal septum during embryologic development. (A) The laryngotracheal diverticulum forms as a ventral outpouching from the caudal part of the primitive pharynx. (B) Longitudinal tracheoesophageal folds begin to fuse toward the midline to eventually form the tracheoesophageal septum. (C) The tracheoesophageal septum has completely formed. (D) If the tracheoesophageal septum deviates posteriorly, esophageal atresia with a tracheoesophageal fistula develops.

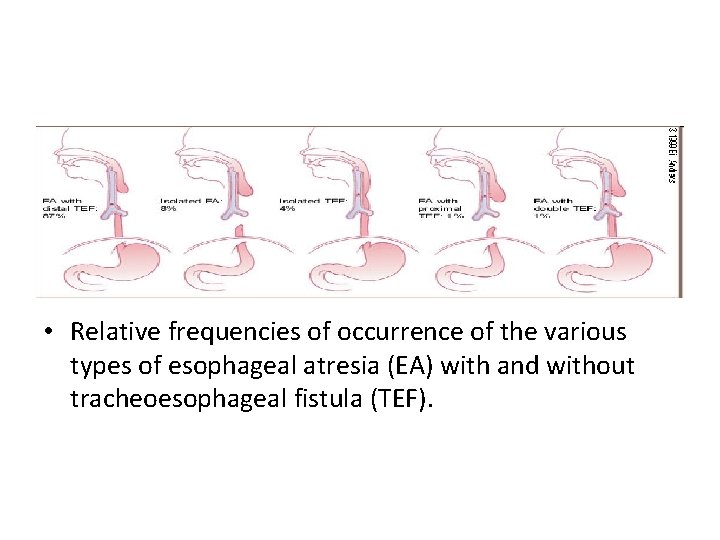
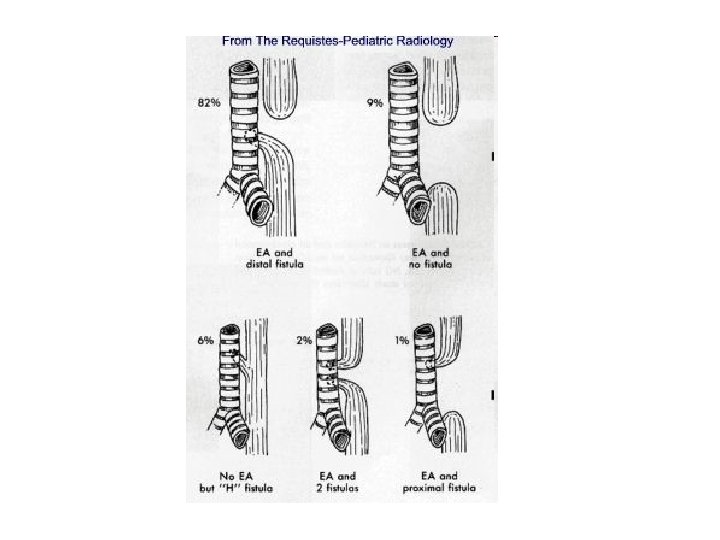
• Relative frequencies of occurrence of the various types of esophageal atresia (EA) with and without tracheoesophageal fistula (TEF).

• Esophageal atresia is characterized by incomplete formation of the esophagus. It is often associated with a fistula between the trachea and the esophagus. Many anatomic variations of esophageal atresia with or without tracheoesophageal fistula have been described 7, 8 (Figure 4). Table 11, 3, 9 -12 provides a summary of the incidence of these variations at multiple worldwide surgical centers. The most common variant of this anomaly consists of a blind esophageal pouch with a fistula between the trachea and the distal esophagus, which is estimated to occur 84 percent of the time. The fistula often enters the trachea close to the


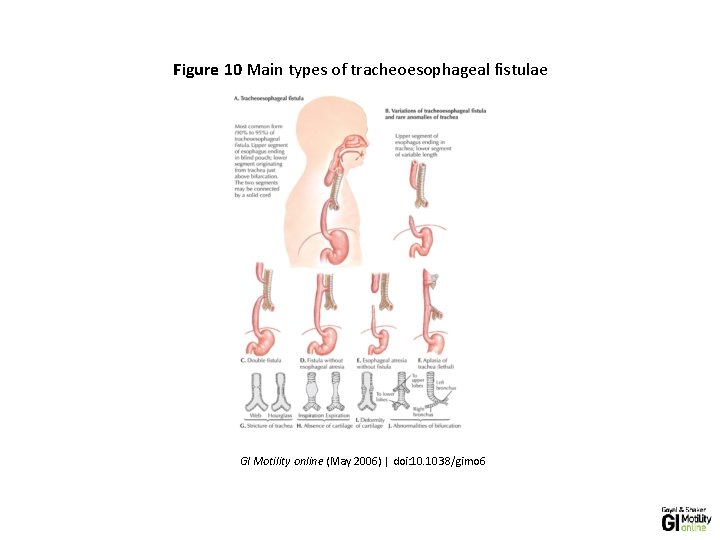
Figure 10 Main types of tracheoesophageal fistulae GI Motility online (May 2006) | doi: 10. 1038/gimo 6

• he figure shows both tracheoesophageal fistula (A-E) and tracheal abnormalities ( F-J). Note that A-E do not correspond with the classification of the type of tracheoesophageal fistula. A shows type C, B type B, C type D, D type E, and E type A tracheoesophageal fistula, respectively. (Source: Netter medical illustration with permission from Elsevier. All rights reserved. )


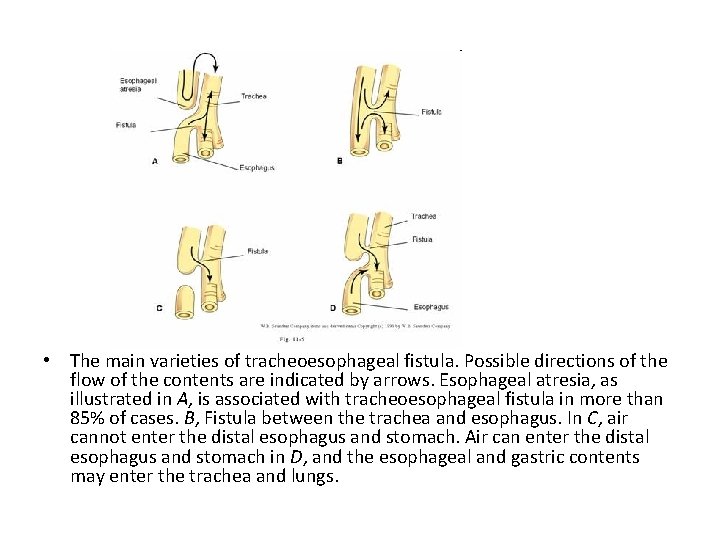
• The main varieties of tracheoesophageal fistula. Possible directions of the flow of the contents are indicated by arrows. Esophageal atresia, as illustrated in A, is associated with tracheoesophageal fistula in more than 85% of cases. B, Fistula between the trachea and esophagus. In C, air cannot enter the distal esophagus and stomach. Air can enter the distal esophagus and stomach in D, and the esophageal and gastric contents may enter the trachea and lungs.

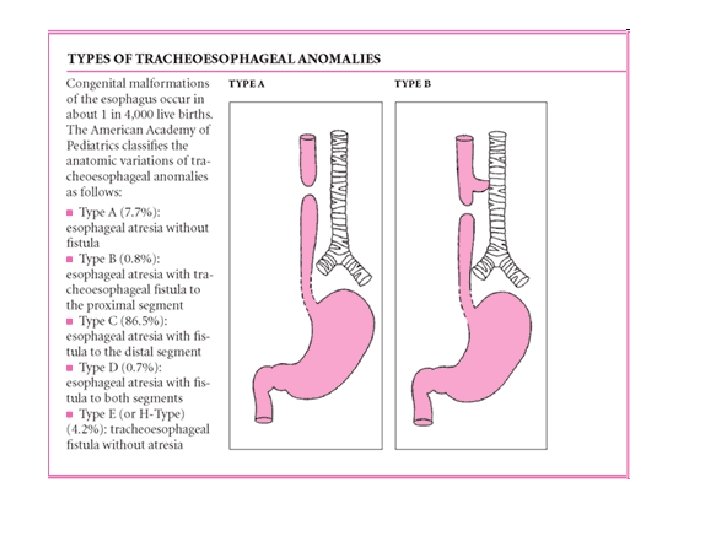
• Classification (Gross's Anatomical Classification) • Type A: Esophageal atresia without tracheoesophageal fistula. • Type B: Esophageal atresia with proximal tracheoesophageal fistula. • Type C: Esophageal atresia with distal tracheoesophageal fistula (most common type) (85%). • Type D: Esophageal atresia with proximal and distal fistula. • Type E: Tracheoesophageal fistula without atresia. (Not shown)