Developing veterinary legislation in a WTO context OIE














- Slides: 29

Developing veterinary legislation in a WTO context OIE Global Conference on Veterinary Legislation 7 -9 December 2010 (Djerba, Tunisia) Melvin Spreij Counsellor Agriculture and Commodities Division

Location: Geneva, Switzerland Established: 1 January 1995 Membership: 153 countries Budget: 185 m Swiss francs, 2008 Secretariat staff: ~650 Head: Pascal Lamy (Director-general) 2

WTO Members 2010 (153) 3

Functions • Negotiate trade rules • Implement trade agreements • Resolve trade disputes • Review national trade policies 4

The basic principles • No discrimination – Most favoured nation principle (MFN) – National treatment principle • Predictability – Respect of tariff “bindings” (goods and services) – Transparency (notification, TPR) • Freer trade (suppression of barriers through negotiations) – Tariff reductions – Prohibition of using quantitative restrictions (quotas) 5

Relevant WTO Agreements • • General Agreement on Tariffs and Trade (GATT) – basic principles Agreement on the Application of Sanitary and Phytosanitary Measures (SPS Agreement) Member states must respect obligations under WTO agreements when developing veterinary legislation 6

Objective of the SPS Agreement? recognizing right to protect human, animal, plant life or health avoiding unnecessary barriers to trade 7

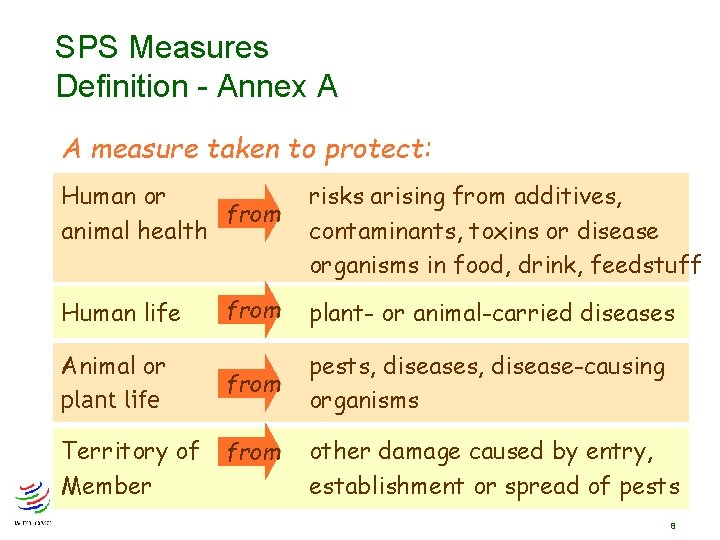
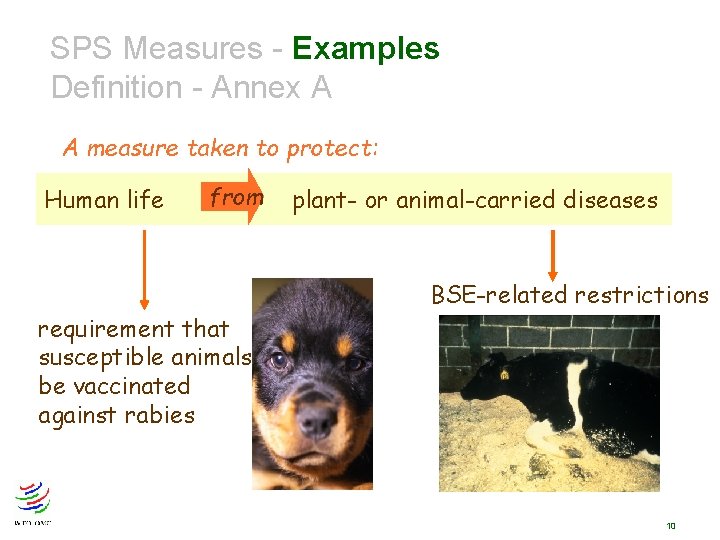
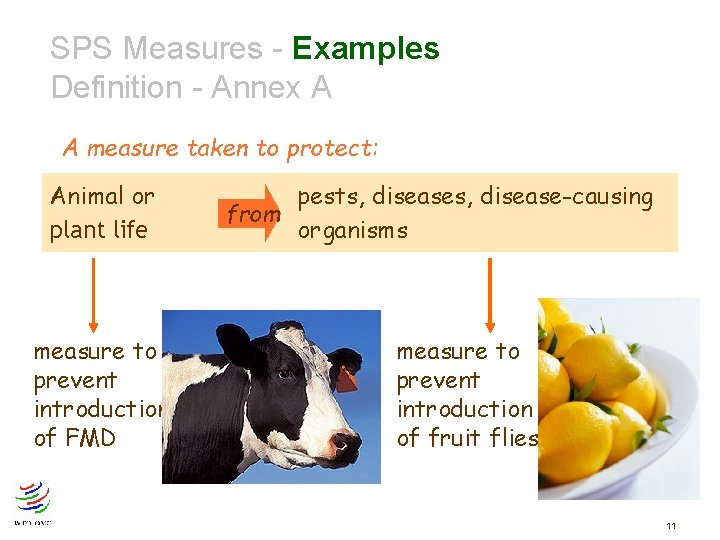
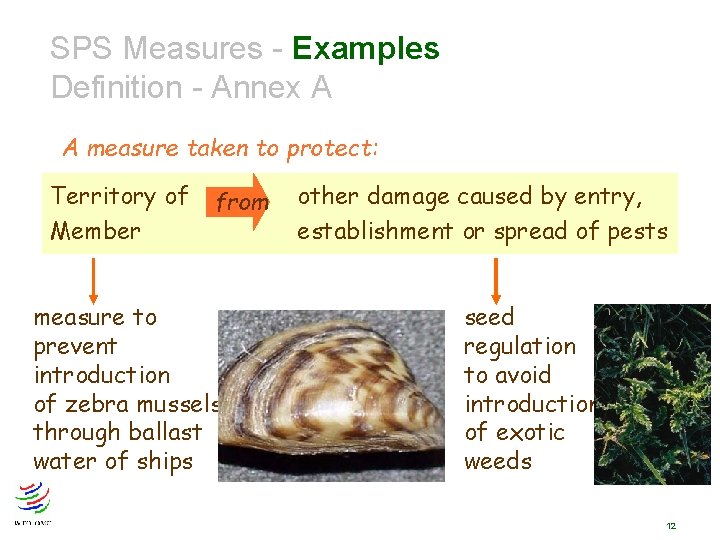
SPS Measures Definition - Annex A A measure taken to protect: Human or from animal health Human life Animal or plant life Territory of Member risks arising from additives, contaminants, toxins or disease organisms in food, drink, feedstuff from plant- or animal-carried diseases from pests, disease-causing organisms from other damage caused by entry, establishment or spread of pests 8

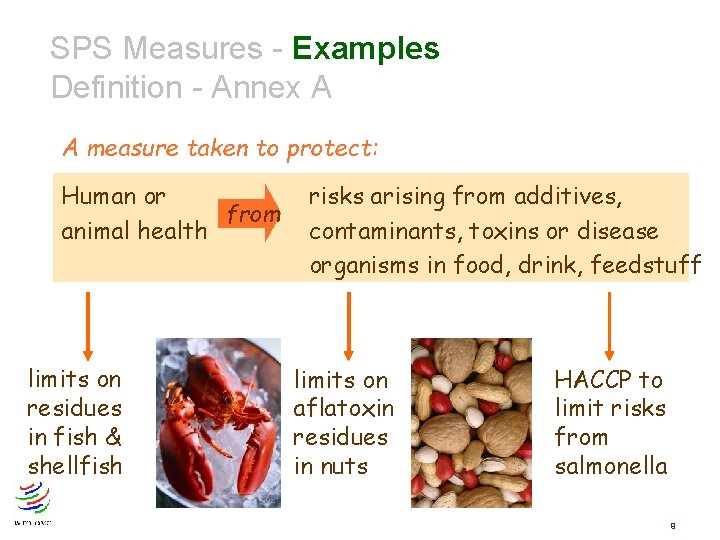
SPS Measures - Examples Definition - Annex A A measure taken to protect: Human or from animal health limits on residues in fish & shellfish risks arising from additives, contaminants, toxins or disease organisms in food, drink, feedstuff limits on aflatoxin residues in nuts HACCP to limit risks from salmonella 9

SPS Measures - Examples Definition - Annex A A measure taken to protect: Human life from plant- or animal-carried diseases BSE-related restrictions requirement that susceptible animals be vaccinated against rabies 10

SPS Measures - Examples Definition - Annex A A measure taken to protect: Animal or plant life measure to prevent introduction of FMD pests, disease-causing from organisms measure to prevent introduction of fruit flies 11

SPS Measures - Examples Definition - Annex A A measure taken to protect: Territory of Member from measure to prevent introduction of zebra mussels through ballast water of ships other damage caused by entry, establishment or spread of pests seed regulation to avoid introduction of exotic weeds 12

SPS measures include all relevant laws, decrees, regulations, requirements and procedures, including inter alia: üproduct criteria üquarantine treatments üproduction and processing requirements ücertification and approval procedures üinspection ütesting 13

Key provisions of the SPS Agreement 1. Non-discrimination 2. Scientific justification • harmonization • risk assessment • consistency • least trade-restrictiveness 3. Equivalence 4. Regionalization 5. Control, inspection and approval procedures 6. Transparency 14

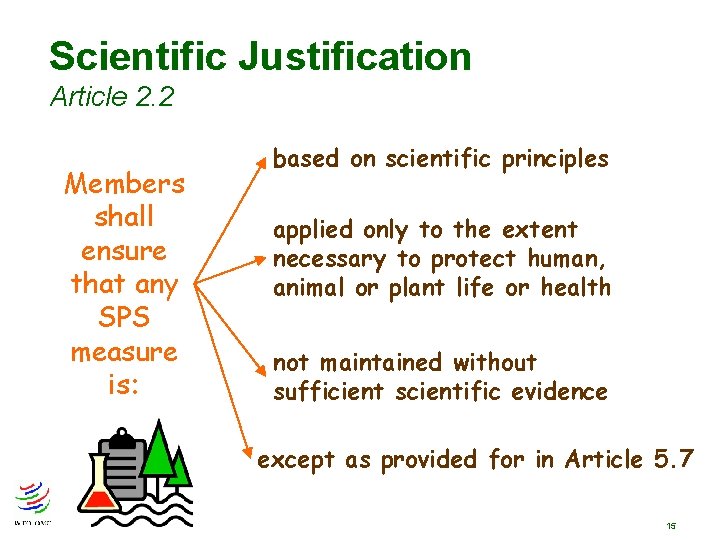
Scientific Justification Article 2. 2 Members shall ensure that any SPS measure is: based on scientific principles applied only to the extent necessary to protect human, animal or plant life or health not maintained without sufficient scientific evidence except as provided for in Article 5. 7 15

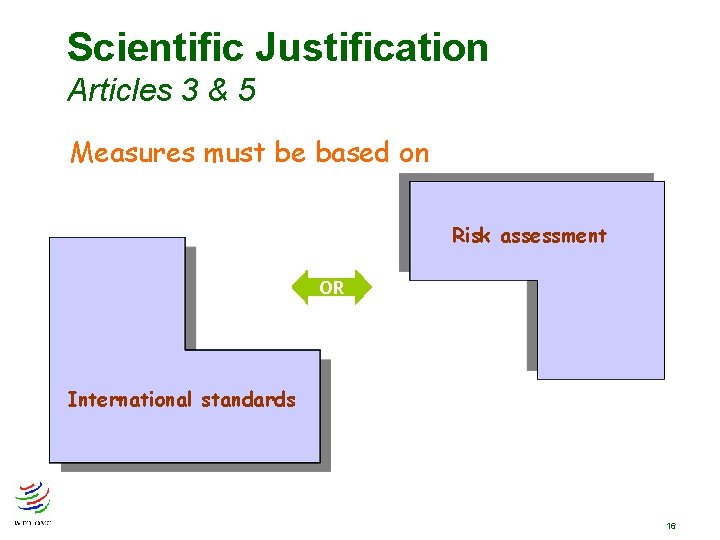
Scientific Justification Articles 3 & 5 Measures must be based on Risk assessment OR International standards 16

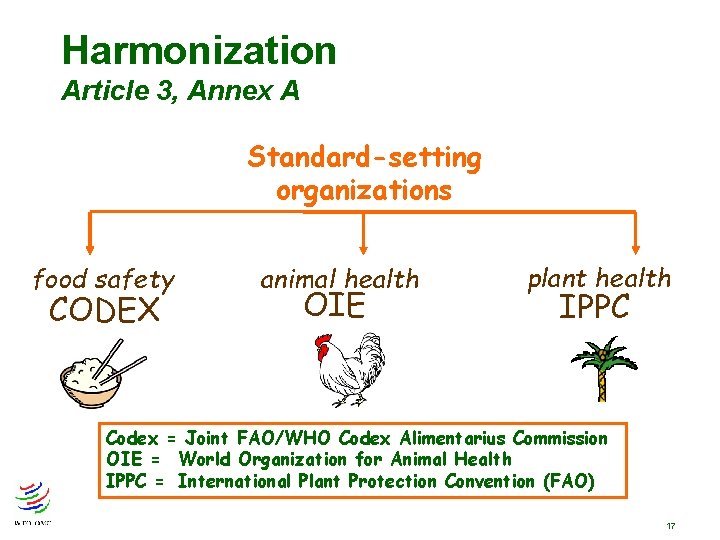
Harmonization Article 3, Annex A Standard-setting organizations food safety CODEX animal health OIE plant health IPPC Codex = Joint FAO/WHO Codex Alimentarius Commission OIE = World Organization for Animal Health IPPC = International Plant Protection Convention (FAO) 17

Risk assessment Members shall ensure that their SPS measures are based on: – an assessment, as appropriate, of the risks to human, animal or plant life or health, – taking into account risk assessment techniques developed by the relevant international organizations Definitions in Annex A: • Food/beverage/feed borne risk • Disease or pest risk 18

Risk assessment - exception Provisional measures, Article 5. 7 Members may provisionally adopt SPS measures üwhen relevant scientific information is insufficient üon the basis of available information In such circumstances, Members shall üseeks to obtain additional information to assess risk üreview the measure within a reasonable period of time 19

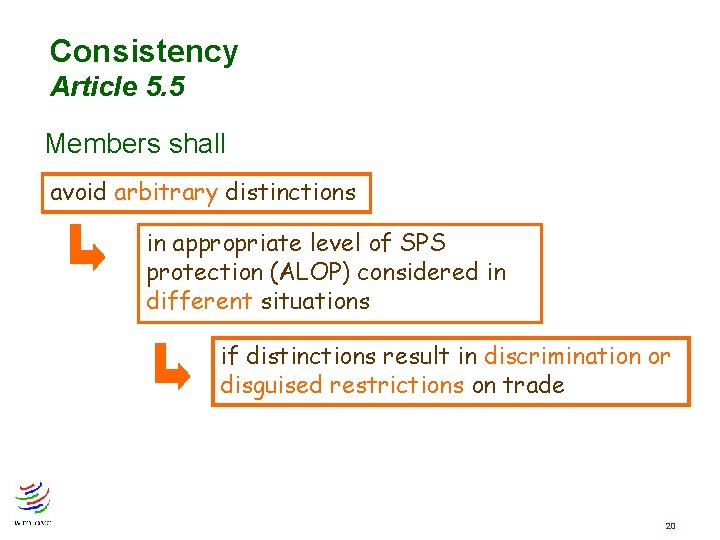
Consistency Article 5. 5 Members shall avoid arbitrary distinctions in appropriate level of SPS protection (ALOP) considered in different situations if distinctions result in discrimination or disguised restrictions on trade 20

Least trade-restrictive – Article 5. 6 • SPS measures not to be more trade restrictive than required to achieve the appropriate level of protection • Alternative measure. . . – – – reasonably available technically and economically feasible significantly less trade restrictive 21

Equivalence Article 4 If the exporting Member objectively demonstrates that its measures achieve the ALOP of the importing country Members shall accept SPS measures of other Members as equivalent SPS Committee Guidelines (G/SPS/19/Rev. 2) 22

Regionalization Article 6 • Adapt SPS measures to characteristics of area (all or part of a country, all or parts of several countries) taking into account • prevalence of diseases or pests • existence of eradication or control programmes • criteria/guidelines developed by OIE, IPPC • Recognize concept of pest- or disease-free areas • Exporters=>proof SPS Committee guidelines (G/SPS/48) 23

Control, Inspection and Approval Procedures - Article 8 and Annex C • No undue delays • Information requirements: limited to what is necessary • No less favourable treatment for imports: – Fees – no discrimination, only to cover costs • Procedure to review complaints 24

Transparency Article 7 & Annex B Members shall establish an Enquiry Point AND designate a Notification Authority notify other Members of new or changed SPS regulations when no international standard exists OR the new regulation is different than the international standard AND regulation may have significant effect on trade 25

When to notify? Regular measures When modifications are still possible (draft text) Allow 60 day comment period!! Provisional measures IMMEDIATELY!! 26

The SPS Committee 3 regular meetings per year – Geneva • Implementation of SPS Agreement • Reviews compliance • Potential trade impacts • Co-operation with technical organizations Codex, OIE and IPPC have observer status 27

Conclusions: • Veterinary legislation should facilitate implementation of provisions of the SPS Agreement and application of relevant guidelines developed by the Committee • New or changed legislation should be notified to the WTO Secretariat in a draft stage • Countries are encouraged to participate actively in the work of the ISSBs and SPS Committee 28

Where to find SPS information? • SPS gateway: http: //www. wto. org/sps • SPS Information Management System (SPS-IMS): http: //spsims. wto. org/ • http: //www. wto. org/ “Docs-on-line” 29